Now - 06:39:06
Cathodic protection: application & standards
Corrosion is a chemical and electrochemical reaction of the metal with the environment, causing damage. It flows at different speeds, which can be reduced. From a practical point of view, the interest is anti-corrosion cathodic protection of metal structures in contact with earth, with water and with the transported media. Especially damaged outer surface of the pipe from the influence of the soil and stray currents.
Inside corrosion depends on the properties of the medium. If it is gas, it needs to be thoroughly cleaned of moisture and aggressive substances: hydrogen sulphide, oxygen etc.
Working Principle
Objects of the process are the electrochemical corrosion environment, the metal and the interface between them. The medium is usually moist soils or water, has good electrical conductivity. At the interface between it and the metal structure is electrochemical reaction. If the current is positive (anode electrode), iron ions pass into the surrounding solution, resulting in the loss of metal mass. The reaction is corrosive. At negative current (cathodic electrode) of these losses can not, because the solution was transferred to the electrons. Method is used in electroplating to be applied to the steel coating of non-ferrous metals.
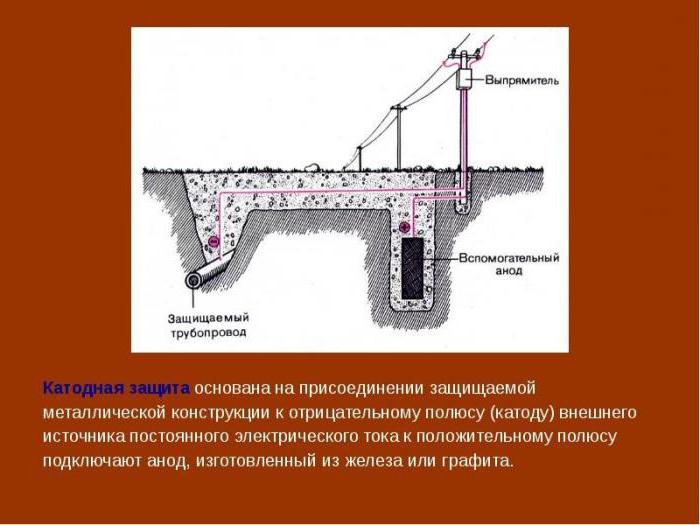
Cathodic corrosion protection is carried out when an object made of iron down the negative potential.
To do this in the ground place the anode electrode and connect the positive potential from the power source. Minus is served on a securable object. A cathode-anode protection leads to active destruction from corrosion of the anode electrode. It should therefore be changed periodically.
Negative effects of electrochemical corrosion
Corrosion of structures can occur from stray currents entering from other systems. They are useful for targets but cause significant harm to nearby structures. Stray currents can spread from the rails of the electrified transport. They pass towards the substation into the pipelines. When leaving they formed anodic areas, causing intense corrosion. To protect used electroking - special drainage currents from the pipeline to their source. There is also a possible cathodic protection of pipelines from corrosion. It is necessary to know the magnitude of the circulating current, which is measured with special devices. 
Recommended
Staff evaluation: system and methods
Personnel Assessment allows you to identify how competent the employees involved in the enterprise, and it is the performance of their work – the most significant factor affecting the efficiency of the company. To clarify the impact of performa...
How to start your own business: important aspects.
Many people, tired of working for someone else, are increasingly thinking about how to start your own business. Someone wants to open a salon, someone store, and someone enough and vegetable stalls. Before you throw in the pool with his head, it is i...
Business activities. its essence and basic functions
The Entrepreneurial activity of the citizen – is undertaken at your own risk and independent activity, which aims to systematically profit through the sale of works, goods, services, use of the property. The citizen engaged in such activities, ...
According to the results of electrical measurements chosen method of protection of the pipeline. Universal remedy is a passive way to isolate pipes from contact with the ground by means of insulating coatings. Cathodic protection of the pipeline refers to the active method.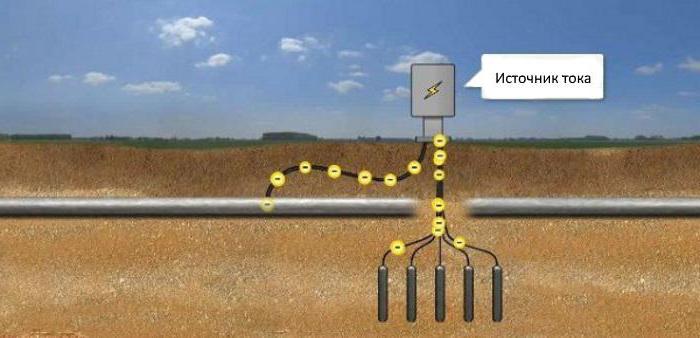
Protection of pipelines
Construction in the ground, protect from corrosion, if you connect the minus of the DC power source, a plus - to the anode electrodes buried nearby in the ground. The current going to the structure, protecting it from corrosion. Thus cathodic protection of pipelines, storage tanks or piping in the soil.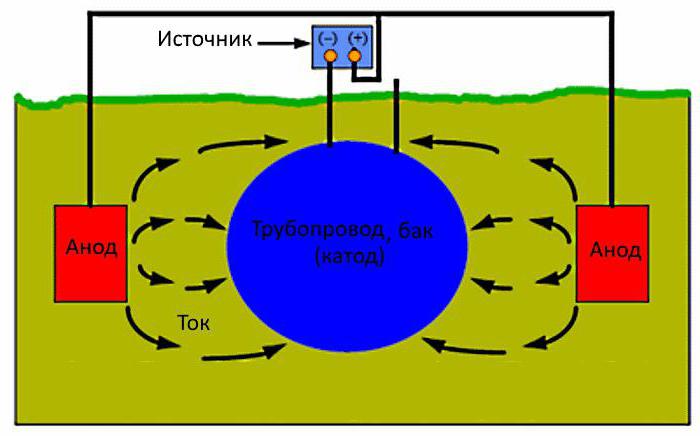
The Anodic electrode will erode, and it should be changed periodically. For tank, filled with water, electrodes placed inside. The liquid is an electrolyte through which the current would flow from the anodes to the surface of the container. The electrodes are well controlled, and easy to replace. In the ground to do it harder.
Power Source
Near oil and gas pipelines, heating networks and water supply systems, which require cathodic protection, set the station from which the voltage is applied to the objects. If they are placed outdoors, the degree of protection should be not less than IP34. For dry areas fits any.
The Station cathodic protection of pipelines and other large structures are rated from 1 to 10 kW.
Their energy parameters primarily depend on the following factors:
- The resistance between ground and the anode;
- Conductivity of the soil;
- The length of the protection zone;
- The insulating effect of the coating.
Traditionally, the Converter for cathodic protection is a transformer installation. Now replaced the inverter with smaller size, better stability and a higher current efficiency. In important areas set controller with functions of regulation of current and voltage, alignment, protective capacities, etc.
The Equipment available on the market in different ways. For the particular needs of an individual design, providing the best conditions of operation.
The Parameters of the current source
To protect against corrosion for the iron protective potential is 0.44 V. In practice, it needs to be more because of the influence of inclusions and surface condition of the metal. The maximum value is 1 V. in the presence of a coating on the metal current between the electrodes is 0.05 mA/m2. If isolation is violated, it increases to 10 mA/m2.
Cathodic protection effective incombination with other methods, because less electricity is consumed. If the surface is paint coating, electrochemical method is protected only where it is violated.
Cathodic protection
- Power supply to serve station or mobile generators.
- The Location of the anode wires depends on the specifics of pipelines. Method of placement may be distributed or concentrated, and placed at different depths.
- Material of the anode is selected with a low solubility to be enough for 15 years.
- Protective field Capacity for each pipeline is calculated. It is not regulated, if the structures are missing a protective coating.
Standard requirements "Gazprom" to the cathodic protection
- Action throughout the life of the protection.
- Protection against atmospheric overvoltage.
- The Location of the station in block-box or in a freestanding vandal-resistant.
- Anode grounded selected in areas with minimal electrical resistance of the soil.
- Characteristics of the transmitter are selected taking into account the aging of the protective coating of the pipeline.
Cathodic protection
Method is a form of cathodic protection connecting the electrodes of more electronegative metal through a conductive medium. The difference lies in the absence of a source of energy. Protector takes corrosion on itself, dissolving in a conductive environment.
After a few years the anode should be replaced, as it is produced.
The effect of the anode increased with the decrease in his transition resistance with the environment. Over time, it may be covered by a corrosion layer. This leads to disruption of the electrical contact. If you put the anode in a mixture of salts, providing the dissolution of corrosion products, the efficiency increases.
The influence of the protector is limited. The range is determined by the electrical resistance of the medium and the potential difference between the anode and cathode.
Cathodic protection is applied in the absence of energy sources or when their use is economically impractical. It is also disadvantageous when used in acidic environments due to the high rate of dissolution of the anodes. Protectors are installed in water, in soil or in a neutral environment. Anodes of pure metals usually do not. The dissolution of zinc is uneven, the magnesium corrodes too quickly, and the aluminum forms a strong film of oxides.
Materials protectors
To protectors possess the necessary performance properties, they are made of alloys with the following alloying additives.
- Zn + 0,025-0,15% Cd+ 0,1-0,5 % Al - protection equipment, located in the sea water.
- Al + 8% Zn +5 % Mg + Cd, In, Gl, Hg, Tl, Mn, Si (percent) - the usage of buildings in flowing sea water.
- Mg + 5-7% Al +2-5 % Zn - protection of small structures in the soil or in water with low concentrations of salts.
Incorrect use of certain types of protectors leads to negative consequences. The anodes of magnesium can be the cause of the cracking equipment due to the development of hydrogen embrittlement.
Joint sacrificial cathodic protection corrosion resistant coatings increases its effectiveness.
Distribution of the protective current is improved and the anodes requires much less. One magnesium anode protects covered with bitumen pipeline to a length of 8 km and uncoated - only 30 meters.
Protection of car body from corrosion
In violation of the coating thickness of the car body can be reduced by 5 years up to 1 mm, i.e. to rust through. Restoration of the protective layer is important, but apart from him there is a way for the complete cessation of the corrosion process using a cathode-cathodic protection. If you turn the body to the cathode, the corrosion of metal is terminated. Anodes can be any conductive surface, located next to: a metallic plate, a ground loop, the body of the garage, a wet road surface. The protection efficiency increases with increasing area of the anodes. If the anode is the road surface, for contact with him used the "tail" of metallizovannoj rubber. It is placed in front of the wheels to better got spray. The "tail" is isolated from the hull.
To the anode connects plus battery via a 1kω resistor and connected in series with it led. On closing the circuit through the anode, when the negative is connected to the body, in normal mode, the led glows faintly. If it burns bright, then the circuit is shorted. The cause must be found and eliminated.
To protect in series in the circuit need to be fused.
When the vehicle is in the garage it is connected to a grounding anode. During the movement of the connection occurs through the "tail".
Conclusion
Cathodic protection is a method of increasing the operational reliability of underground pipelines and other structures. This should take into account its negative impact on neighboring pipelines from the effects of stray currents.
Article in other languages:
AR: https://tostpost.com/ar/business/11592-cathodic-protection-application-standards.html
BE: https://tostpost.com/be/b-znes/20763-katodnaya-abarona-prymyanenne-standarty.html
DE: https://tostpost.com/de/business/20771-kathodischer-schutz-anwendung-und-standards.html
ES: https://tostpost.com/es/negocio/20785-protecci-n-cat-dica-aplicaci-n-de-normas-y.html
HI: https://tostpost.com/hi/business/11603-cathodic-protection-application-standards.html
JA: https://tostpost.com/ja/business/11603-cathodic-protection-application-standards.html
KK: https://tostpost.com/kk/biznes/20755-katodty-or-au-oldanu-zh-ne-standarttar.html
PL: https://tostpost.com/pl/biznes/20724-ochrona-katodowa-zastosowanie-i-standardy.html
PT: https://tostpost.com/pt/neg-cios/20723-prote-o-cat-dica-aplica-o-e-normas-de.html
TR: https://tostpost.com/tr/business/20764-katot-koruma-uygulama-ve-standartlar.html
UK: https://tostpost.com/uk/b-znes/20751-katodniy-zahist-zastosuvannya-standarti.html
ZH: https://tostpost.com/zh/business/12416-cathodic-protection-application-standards.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
As a gander to distinguish from goose: appearance, behavioral and anatomical characteristics
the Breeding of geese is today one of the most profitable types of business. Not every aspiring poultry farmer knows how to distinguish the gander from the goose. Meanwhile, it is very important in order to achieve the optimum rat...
How to order special equipment.
Today, many people wonder: where and how to order the equipment? So as to purchase special equipment for the construction of an object or other types of work is quite expensive. is currently in any city, there are many...
Billionaire Samvel Karapetyan Sarkisovich
Samvel Karapetyan Sarkisovich is a well - known Russian businessman and philanthropist of Armenian origin. Want to know more about this businessman, his life and work more? Welcome to this article!Educationa Future entrepreneur wa...
SAM "Shtil": technical description and comparison with analogues
Modern Russian weapons in the form of SAM "Shtil" is a multi-channel launcher that focuses on naval basing, it is equipped with a vertical launch. The system is designed to implement a circular defense of the ship and reflection e...
Steeplejack work: features of industrial mountaineering
a Glance at any modern city from a bird's eye view, one can marvel at the saturation of its infrastructure. Almost wall to wall with residential houses can be the case a variety of institutions, territories of industrial enterpris...
Polymer - what is it? The production of polymers
it's Amazing how diverse the surrounding objects and the materials from which they are made. Earlier, around the XV-XVI centuries, the main materials were metal and wood, later glass, all porcelain and faience. But today's age is ...






















Comments (0)
This article has no comment, be the first!