Thermodynamics and heat transfer. Methods of heat transfer and calculation. Heat transfer is...
Today we will try to find the answer to the question “the Heat transfer is...?”. In the article, consider what is the process by which species exist in nature, and also learn what is the relationship between heat transfer and thermodynamics.
Definition

Heat transfer is a physical process, the essence of which is to transfer thermal energy. The exchange occurs between two bodies or their system. The prerequisite is the transfer of heat from more heated to less heated bodies.
Features
Heat transfer is the same type of phenomena, which may occur by direct contact, and the presence of separating walls. In the first case everything is clear, in the second as the barriers can be used body, materials, environment. Heat transfer will occur in cases where a system consisting of two or more bodies, is not in a state of thermal equilibrium. That is, one object has a higher or lower temperature compared to others. Here then is the transfer of thermal energy. It is logical to assume that it will be completed when the system returns to the state of thermodynamic or thermal equilibrium. The process is spontaneous, what can you tell us the second law of thermodynamics.
Types
Heat transfer is a process which can be divided into three ways. They will have a basic nature, since inside them there are these subcategories have their own distinctive features along with the General laws. Today it is accepted to allocate three types of heat transfer. This conduction, convection, and radiation. Let's start with the first.
Methods of heat transfer. Thermal conductivity.

The So-called property of one or other material body to perform the energy transfer. However, she is transferred from the hotter part to the colder than that. The basis of this phenomenon is the principle of the chaotic movement of molecules. This so-called Brownian motion. The more the body temperature, the more it moving molecules because they have greater kinetic energy. In the process of heat conduction involving electrons, molecules, atoms. It is carried out in the bodies, the different parts which have different temperatures.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
If the substance is able to conduct heat, we can speak about the presence of quantitative characteristics. In this case, it is the role of the thermal conductivity. This indicates how much heat will pass through the individual indicators of length and area per unit time. The body temperature will change by exactly 1.
Previously it was thought that the exchange of heat in different bodies (including the heat transfer of enclosing structures) stems from the fact that from one body part to another flows the so-called caloric. However, the signs of its actual existence were never found, and when the molecular-kinetic theory has developed to a certain level, all about the caloric and forgot to think, because the hypothesis was untenable.
Convection. Heat transfer of water

Under this method of thermal energy exchange is a transfer using internal threads. Let us imagine a kettle with water. As you know, more heated air flow rising upward. And the cold, heavier, fall down. So why water should it be any different? It's all exactly the same. And in the course of this cycle all the layers of water, no matter how many, will heat up until a state of thermal equilibrium. Under certain conditions, of course.
Radiation
This method consists in the principle of electromagnetic radiation. It arises from internal energy. To go down much in the theory of heat radiation will not, just note that the reason for this is the device charged particles, atoms and molecules.
Simple tasks on the thermal conductivity
Now let's talk about how looks in practice the calculation of heat transfer. Let's choose a simple task related to the amount of heat. Suppose we have a mass of water equal to half a kilogram. Initial water temperature-0 degrees Celsius, the end – 100. Find the amount of heat expended by us for heating this mass of matter.
For this we need the formula Q = cm(t2-t1), where Q-the quantity of heat c-specific heat of water, m-mass of the substance, t1 – primary, t2 – the final temperature. For water the value of c is tabular in nature. Specific heat capacity is equal to 4200 j/kg*C. Now we substitute these values into the formula. Get the amount of heat will be equal to 210000 joules or 210 kJ.
First law of thermodynamics

Thermodynamics and heat transfer are linked by some laws. They are based on the knowledge that the change in internal energy within the system can be achieved by two ways. First - to perform mechanical work. Second – a specific amount of heat. Based on this principle, incidentally, the first law of thermodynamics. Here is his formulation: if the system was this some amount of heat, it will be spent onthe Commission of work on external bodies, or the increment of its internal energy. Mathematical notation: dQ = dU + dA.
Pros or cons?
Absolutely all quantities that are included in the mathematics of the first law of thermodynamics, can be written as with a “plus” and mark “minus”. Moreover, their selection will be dictated by the process conditions. Suppose that the system receives a certain amount of heat. In this case, the body in her heat. Therefore, the gas expansion and, therefore, work is being performed. In the end the values will be positive. If the quantity of heat taken away, the gas cools down over him is the work. The values take the opposite meaning.
An Alternative formulation of the first law of thermodynamics
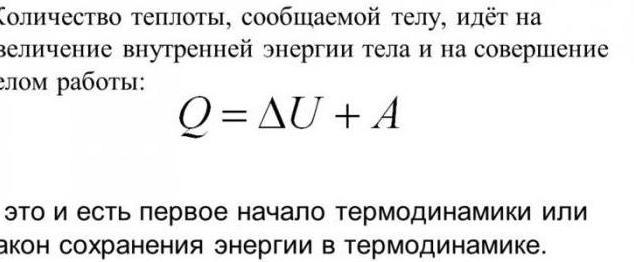
Suppose we have a regular engine. In it the working body (or system) make a circular process. It is called a cycle. In the end, the system returns to the original state. It would be logical to assume that in this case, the change in internal energy is zero. It turns out that the amount of heat will be equal to the work. These provisions allow us to formulate the first law of thermodynamics is different.
From this we can understand that nature can not exist a perpetual motion machine of the first kind. That is, a device that does work in greater numbers compared to the received energy. The actions must be performed periodically.
First law of thermodynamics for processes
Consider first the isochoric process. When the volume remains constant. Hence, the volume change will be zero. Therefore, the work will also be equal to zero. Discard this term from the first law of thermodynamics, and then have the formula dQ = dU. Then, in the isochoric process all the heat supplied to the system goes into increasing the internal energy of the gas or mixture.
Now let's talk about Isobaric process. Constant it still has pressure. The internal energy will change in parallel with the performance of work. This is the original formula: dQ = dU + pdV. We can easily calculate the committed work. It will be equal to the expression uR(T2-T1). By the way, this is the physical meaning of the universal gas constant. In the presence of one mole of gas and the temperature difference, one Kelvin component, the universal gas constant is equal to the work done in Isobaric process.
Article in other languages:

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
"The allure of the seas" - the largest airliner in the world
"the Allure of the seas" is the largest airliner in the world today. To him those were considered "the oasis of the seas". Interestingly, the difference between them is only... 5 inches! In fact, this ship's twin, but the palm is ...
The principle of relativity as the Foundation of the theory of relativity
Presented to the scientific community at the beginning of the last century the theory of relativity made a splash. Its author, A. Einstein, for decades identified the main areas of physics research. But do not forget that the Germ...
Novosibirsk universities - prepares graduates
By population, the capital of the Siberian Federal district, Novosibirsk is the third city in Russia, which is a major business, industrial, cultural, scientific and commercial center of Siberia. Many Novosibirsk universities - un...
What the sea washes Greece? Find out!
Greece – one of the most unique countries in the world. . Developed a unique ancient culture, there was born of ancient gods and mythical heroes. In our days the territory of this country is a historical and tourist centre, ...
The design of the title page is an important and integral part of the course work, essay, report
Making a title page for any research paper (including abstract) must meet certain rules and norms of GOST. Title page – this is the first thing the tutor will pay attention.in addition, to make their demands may school. It i...
The anomalous zone. Logic is unusual
On the surface of the planet Earth a lot of areas, causing not only a lot of questions from ordinary citizens, but also puts in a difficult position of scientists. While the hearsay attributed to these areas the title of black spo...






















Comments (0)
This article has no comment, be the first!