Now - 14:45:24
Real gases: deviations from ideality
The Term “real gases” among chemists and physicists called such gases, which properties are the most directly dependent on their intermolecular interactions. Although in any workshop manual you can read that one mole of these substances in normal conditions and steady state occupies a volume of approximately 22,41108 L. This statement is true only for so-called "ideal" gases, which, in accordance with the Clapeyron equation, are not the forces of mutual attraction and repulsion of molecules, and the last occupied volume is negligibly small quantity.

Of Course, such substances do not exist, therefore all these arguments and calculations are purely theoretical orientation. But the real Gaza, which in varying degrees, deviate from the laws of perfection, are very frequent. Between the molecules of such substances are always present forces of mutual attraction, which means that their volume is somewhat different from that shown perfect model. All real gases have different degree of deviation from ideality.
But here there is a very clear trend: the more the boiling point of a substance is close to zero degrees Celsius, the stronger the connection will differ from the ideal model. The equation of state of real gas, owned by the Dutch physicist Johannes Diderik van der Waals, was derived to them in 1873. In this formula having the form (p + n2A/V2) (V-nb) = nRT, introduced two very significant amendments compared with the Clapeyron equation (pV= nRT), determined experimentally. The first of them takes into account the forces of molecular interaction, which influences not only the type of gas, but also its volume, density and pressure. The second amendment is determined by the molecular weight of the substance.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
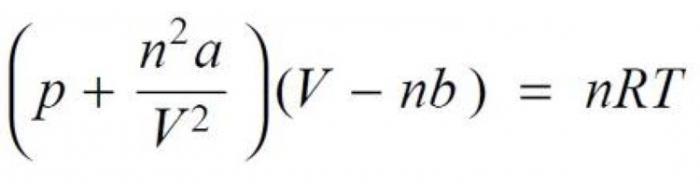
The Most significant role adjustments acquire data at high pressure gases. For example, for nitrogen at the rate of 80 ATM. calculations will differ from the ideal by about five percent, and with increasing pressure up to four atmospheres, the difference will reach one hundred percent. It follows that the laws of the ideal gas model is very approximate. Deviation from them is both quantitative and qualitative. The first is shown that the Clapeyron equation applies to all real gases approximate. Retreat the same quality of character is much more deep.
Real gases can be converted in the liquid and in the solid state, which would be impossible if strict adherence to the Clapeyron equation. Intermolecular forces acting on these substances, lead to the formation of different chemical compounds. Again, this is impossible in a theoretical ideal gas system. Educated thus the relation is called chemical or valence. In the case when the real gas is ionized, it begins to show the Coulomb force of attraction, which determines the behavior of, for example, plasma, representing an ionized quasi-neutral substance. This is especially true in light of the fact that plasma physics is today a vast, rapidly developing scientific discipline with an extremely wide application in astrophysics, the theory of propagation of radio wave signals, the problem of controlled nuclear and thermonuclear reactions.
The Chemical bond in real gases by their nature do not differ from the molecular forces. And those and others by and large are reduced to the electric interaction between elementary charges, all of which are built of atomic and molecular structure of the substance. However, a complete understanding of the molecular and chemical forces became possible only with the emergence of quantum mechanics.
It Is recognized that not every state of matter compatible with the equation the Dutch physicist, can be implemented in practice. This requires also the factor of their thermodynamic stability. One of the important conditions for such stability of the substance is that in the isothermal equation for pressure must be strictly observed trend of decrease in the total volume of the body. In other words, increasing values of V all the isotherms of a real gas should steadily fall. Meanwhile, on the isothermal charts of van der Waals forces below the critical temperature marks are observed rising areas. Of the point lying in such zones correspond to the unstable state of matter, which in practice cannot be realized.
Article in other languages:
AR: https://tostpost.com/ar/education/6823-real-gases-deviations-from-ideality.html
BE: https://tostpost.com/be/adukacyya/12196-real-nyya-gazy-adh-lenne-ad-deal-nasc.html
DE: https://tostpost.com/de/bildung/12198-reale-gase-abweichung-von-der-idealit-t.html
ES: https://tostpost.com/es/la-educaci-n/12205-real-de-gaza-el-rechazo-de-la-ideal-nosti.html
HI: https://tostpost.com/hi/education/6829-real-gases-deviations-from-ideality.html
JA: https://tostpost.com/ja/education/6826-ideality.html
KK: https://tostpost.com/kk/b-l-m/12199-na-ty-gazdar-auyt-u-ideal-nosti.html
PL: https://tostpost.com/pl/edukacja/12190-prawdziwe-gazy-odchylenie-od-idealno-ci.html
PT: https://tostpost.com/pt/educa-o/12183-real-gases-desvio-de-ideal-nosti.html
TR: https://tostpost.com/tr/e-itim/12201-ger-ek-gazlar-sapma-ideal-nosti.html
UK: https://tostpost.com/uk/osv-ta/12194-real-n-gazi-v-dhilennya-v-d-deal-nost.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
The Huguenot - who is this? The Huguenots and Protestants. The Huguenots in France
In the middle of the sixteenth century, the monarchy in France was going through hard times. Italian war, which ended in defeat, led to a severe crisis of power and economy. French feudal lords, seeking high positions, new land an...
The most famous travellers and their discoveries
Travel has always lured people, but before they were not only interesting but also very challenging. The territory was not explored, and going to a way, everyone became a researcher. What travellers are the most famous and what ex...
Climate Chile: features and advantages
Chile – a country that has become unique thanks to its borders. Externally, it resembles an elongated strip, which is incredibly narrow and very long. For this reason, the climate of Chile is so diverse because the territory...
For half century in biology is well established the classification division of all plants into shade-tolerant and light-loving. These differences can be observed in the natural change of tree species or as a result of the fires. I...
What is that language? The problem of profanity
the Usual spoken language may be unacceptable, just people don't ask enough. The result is an adult one can only wonder where children learn "bad words" and why they are to such a degree attractive. What is profanity, wh...
The basic characteristics of a living organism. The main signs of wildlife
Modern science divides all of nature into animate and inanimate. At first glance, this division may seem simple, but sometimes quite difficult to decide whether a particular natural object is really alive or not. Everyone knows th...





















Comments (0)
This article has no comment, be the first!