Now - 14:23:06
The atom model of Thomson and Rutherford briefly
In the days of the Ancient Greek philosophers knew about the internal structure of matter. And the first model of the structure of atoms appeared at the beginning of XX century. Hypothesis J. Thomson was not accepted by the scientific community that time is critical – because until it had already been put forward various theories about what is inside the smallest particles of matter.

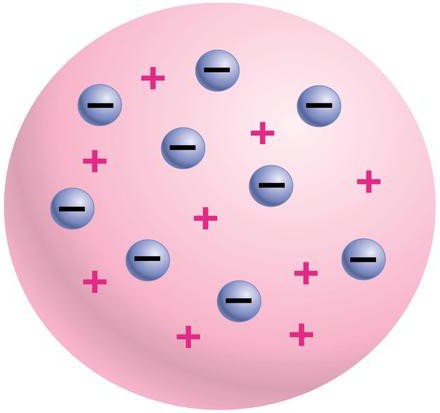
“cookie”, or model Thomson
Until the nineteenth century, scientists assumed that the atom is indivisible. But everything changed after Joseph Thomson in 1897, discovered the electron – it became clear that the scientists were wrong. Both the atom model of Thomson and Rutherford were put forward at the beginning of the last century. First, a model of W. Thomson, which suggested that the atom is a lump of matter with a positive electrical charge. Inside this bundle are uniformly distributed electrons – that is why this model was called “cake”. Because according to it the electrons in matter are like the raisins in the cake. Other unofficial model name – “cookie”.

Merits of John. Thomson
This model was developed more details of John. John. Thomson. Unlike W. Thomson, he assumed that the electrons in an atom are located strictly on the same plane, which is a concentric ring. Despite the equal importance of the atom models of Thomson and Rutherford to science of the time, it is worth noting that George. Thomson, among other things, for the first time proposed a method of determining the number of electrons inside the atom. His method was based on scattering x-rays. John. Thomson suggested that electrons are the particles that should be in the centre of the dispersion of the rays. In addition, Thomson was the scientist who discovered electrons. In modern schools with the study of his discovery starts a course in quantum mechanics.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Cons of the theory of Thomson
However, compared to the model of the Rutherford model of the atom Thomson had one major drawback. She could not explain the discrete nature of radiation atom. It was impossible with it to say anything about the reason for the stability of the atom. It was finally disproved, when the user performed the famous experiments of Rutherford. Model of the atom Thomson had no less value to science of the time than other hypotheses. Be aware that all these models available at that time, was purely hypothetical.
Features of the experience of Rutherford
In the years 1906-1909 G., Geiger, E. Mansinam and E. Rutherford had conducted experiments in which alpha particles were subjected to dispersion on the surface of gold foil. Briefly the atom model of Thomson and Rutherford are described as follows. In the model of Thomson electrons in an atom are distributed unevenly, and in the theory of Rutherford – revolve in concentric planes. A distinctive factor in the experience of Rutherford was using alpha particles instead of electrons. Alpha particles, unlike electrons, had much greater mass, and did not undergo significant variations when faced with the electrons. Therefore, scientists were able to record only the collisions that occurred with the positively charged part of an atom.

Role of the opening of Rutherford
This experience was crucial for science. With it, scientists have been able to get answers to those questions that remained a mystery to the authors of the various models of the atom. Thomson, Rutherford and Bohr, though possessing the same base, still made several different contributions to science – and the results of the experiments of Rutherford in this case was striking. Their results were the exact opposite of what scientists expected to see.
Most of the alpha particles passed through the foil sheet in a straight (or almost straight) paths. However, the trajectory of some alpha particles were deflected at significant angles. And it was evidence that the atom was education with a very high density and had a positive charge. In 1911, based on experimental data was proposed the model of atomic structure of Rutherford. Thomson, the theory of which had hitherto been regarded as dominant, in this time continued to work in the Cavendish laboratory of the University. Until the end of his life, the scientist continued to believe in the existence of a mechanical ether, despite all the progress in scientific research of that time.
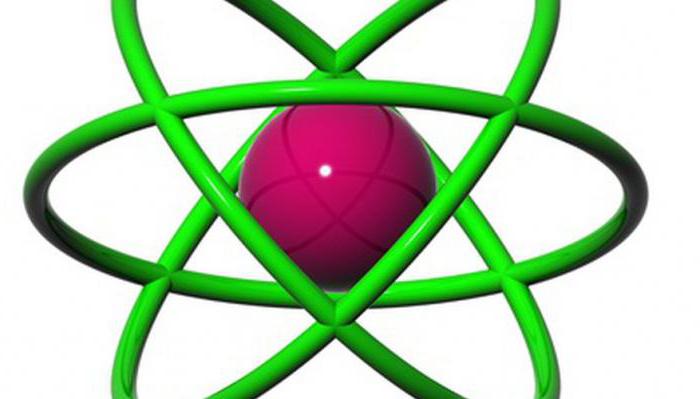
Planetary model Rutherford
Summarizing the results of the experiments, Ernest Rutherford put forward the main concepts of his theory: according to it, the atom consists of a heavy and dense nucleus is very small; around this nucleus are located the electrons in continuous motion. The radii of the orbits of these electrons is also small: they amount to 10-9 m. This model was called "planetary" for its resemblance to the model of the Solar system. In it the planets move in elliptical orbits around a huge and massive center withattraction – the Sun.
Electrons rotate in an atom with such a huge speed that form around the surface of the atom is something like a cloud. According to the theory of Rutherford, the atoms are arranged apart at a distance that allows them to adhere to each other. Because around each of them there is a negatively charged electron shell.
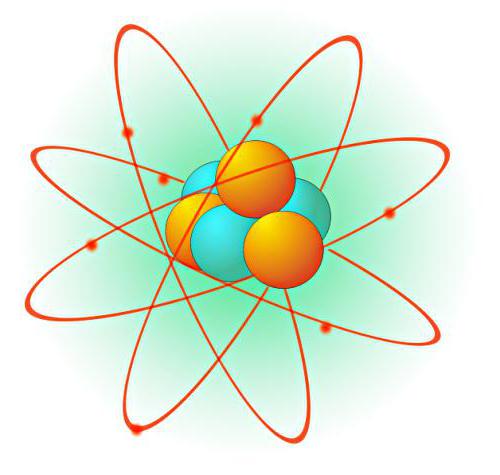
The atom Model of Thomson and Rutherford: the basic differences
What are the main differences between the two major theories of atomic structure? Rutherford assumed that in the center of the atom is a nucleus with a positive electric charge and the volume of which, in comparison with the size of the atom is negligible. Thomson also implied that the whole atom is the formation of high density. The second main difference was the understanding of the position of electrons in the atom. According to Rutherford, they rotate around the nucleus, and their number is approximately equal to ½ the atomic weight of a chemical element. In the theory of Thomson the electrons inside an atom is not evenly distributed.
Cons of the theory of Rutherford
However, despite all the advantages, at that time the theory of Rutherford contained one important contradiction. According to the laws of classical electrodynamics, an electron orbiting the nucleus should continuously emit the portions of electric energy. Because of this, the radius of the orbit on which the moving electron was continuously emitting electromagnetic radiation. According to this view, the lifetime of the atom should be negligible.
Usually when we talk about the opening of the internal structure of the atom, mention the names of Thomson and Rutherford. The experiments of Rutherford, model of atom which is now known to every student of physics and mathematics departments in the universities, currently is part of the history of science. When Rutherford made his discovery, he exclaimed: “Now I know how it looks like an atom!” in reality, However, he was wrong, because the true picture was known to scientists much later. Although the model of Rutherford and have been subjected to over time, significant adjustments, its meaning has remained unchanged.
The Bohr Model
However, in addition to models of the atom Thomson and Rutherford, there was one theory that explained the internal structure of these smallest particles of matter. It belongs to Niels Bohr-Danish physicist who proposed the explanation in 1913. According to his model, the electron in the atom does not obey the standard physical laws. What Bohr was the scientist who introduced in science the concept of the ratio between the radius of the orbit of the electron and its velocity.
In the process of creating his theory Bohr based his model of Rutherford, however, has subjected her to considerable refinement. Models of atoms Bohr, Rutherford and Thomson now can seem a bit simple, but they formed the basis of modern ideas about the internal structure of the atom. It is now accepted quantum model of the atom. Despite the fact that quantum mechanics cannot describe the motion of the planets of the Solar system, the notion of orbit has still remained in the theories describing the internal structure of the atom.
Article in other languages:
AR: https://tostpost.com/ar/education/2205-the-atom-model-of-thomson-and-rutherford-briefly.html
BE: https://tostpost.com/be/adukacyya/3879-madel-atama-tomsana-rezerforda-koratka.html
DE: https://tostpost.com/de/bildung/3879-atommodell-von-thomson-und-rutherford-kurz.html
HI: https://tostpost.com/hi/education/2205-the-atom-model-of-thomson-and-rutherford-briefly.html
JA: https://tostpost.com/ja/education/2204-the-atom-model-of-thomson-and-rutherford-briefly.html
PL: https://tostpost.com/pl/edukacja/3883-model-atomu-thomsona-i-rutherforda-kr-tko.html
PT: https://tostpost.com/pt/educa-o/3880-o-modelo-do-tomo-de-thomson-e-o-de-rutherford-breve.html
TR: https://tostpost.com/tr/e-itim/3887-modeli-atomun-thomson-ve-rutherford-k-saca.html
UK: https://tostpost.com/uk/osv-ta/3883-model-atoma-tomsona-rezerforda-korotko.html
ZH: https://tostpost.com/zh/education/2387-the-atom-model-of-thomson-and-rutherford-briefly.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Tungsten - what is it? The degree of oxidation of tungsten. The scope of tungsten
Tungsten-a chemical element, atomic number of which is equal to 74. This heavy metal from steel-gray to white, featuring high strength, making it in many cases is simply irreplaceable. The melting point of he is higher than any ot...
The average depth of the Arctic ocean, the bottom topography and climate
the Smallest representative of the earth's oceans-the Arctic. It covered the territory of the North pole and borders on different sides of continents. The average depth of the Arctic ocean is 1225 meters. He is the most shallow of...
The art and science. Figures of science and art
If you look at the way in which humanity has passed, we can talk about that for a representative homo sapiens has always been the main three tasks: to survive, to learn and create. If the first question does not arise at all, othe...
The population of Austria: especially, density and strength
Austria is a European Federal state, which is one of the richest in the world. The area of the country is nearly 84 thousand square kilometers. Largest cities – Vienna, Innsbruck, Graz, Salzburg and Linz. The German language...
Remember the word "communiqué" is not too difficult
In the Russian language there are unusual words (mainly borrowed from other languages) that are not only difficult to pronounce but also nearly impossible to immediately determine their gender and number. One of these obscure word...
Territory, the capital and the population of Abkhazia
Abkhazia is a small country but with a very interesting history and a rich heritage.Wherethe Territory of a state located in the North-West Caucasus. It has borders with two countries-Georgia and Russia. Between the rivers Psou an...





















Comments (0)
This article has no comment, be the first!