Now - 19:45:31
Qualitative reactions for alkenes. Chemical properties and structure of alkenes
What are the qualitative reactions for alkenes? In order to answer this question, we must first understand what is the feature of representatives of this class.
Feature class
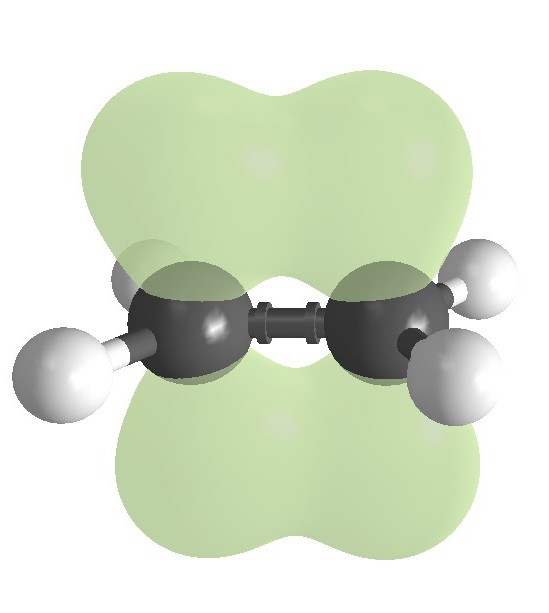
For starters, let's analyze the structure of alkenes. Unsaturated hydrocarbons are called compounds having the General formula CnH2n. The structure of the linear alkenes, but their structure in addition to single (simple) connection is present and complex (double bond).
Nomenclature
Before to characterize the qualitative reactions for alkenes, stop about their names. The sequence of actions is similar to the alkanes class, but there are some distinctive options, which should stay separate. First we need the proposed structure to find the longest carbon chain that includes the double bond.
Next, the atoms are numbered from the side on which is located the double bond closer to the beginning of the chain. If in a molecule are hydrocarbon radicals (other variants of the substituents), in this case, be sure to indicate the number of their position. If you have multiple identical radicals used by specifying prefix. Then called itself the main chain, using the suffix-EN. A requirement (in addition to ethylene and propene) is an indication of the number of the position of multiple bonds.

Isomerism
Qualitative reactions for alkenes associated with the location in the molecules of the double bond. This dependence suggests a more detailed study of the issue concerning the isomerism of unsaturated hydrocarbons. Like other representatives of the hydrocarbons, the molecules of alkenes are structural isomers. For example, substance composition С4Н8 can portray butene and 2-methylpropene.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
In addition, the representatives of this class of unsaturated hydrocarbons and isomerism of double bond position. From its location depend on some chemical properties of this class.
Among the distinctive types of isomerism not possessed by other members of the C x h y, we note the geometric (spatial structure). Depending on the rotation at the double bond of an alkene molecule fragments, it is possible to obtain CIS - and TRANS - isomers. Interclass isomerism alkenes connects with cycloalkane. Such a variety of structures, typical for the homologous series ethylene, determines their basic chemical properties.

Chemical properties
Qualitative reactions for alkenes is their interaction with aqueous solutions of Halogens. These include bromine, iodine, chlorine. As a result of this chemical reaction is cleavage of double bonds, the accession of halogen atoms. As a feature of this interaction is seen discoloration of iodine (bromine) water. Qualitative reactions on the double bond of alkenes is their reaction with water solution of potassium permanganate in an acidified environment.
As products of this reaction are sulfate and organic product. It was a reaction with an oxidizing agent and a solution of Halogens is qualitative reactions for alkenes. Hydrogenation is not required, it will receive the corresponding limit hydrocarbons (alkanes). As mandatory conditions of their occurrence stands high temperature, and the use of a catalyst.
The Activity of alkenes is much more than the paraffins. The reason is the presence between the carbon atoms at the double bond is not too strong bonds.
During the combustion of any hydrocarbon composition CnH2n in oxygen is observed the allocation of water vapor, carbon dioxide, and the formation of a sufficient amount of heat. Qualitative reactions to alkenes can be used for the selection of these compounds among other substances.
The hydration Reaction (water connections) is for asymmetrical alkenes according to Markovnikov rule. According to him the accession of a hydrogen atom to the carbon, which contains more hydrogen. The hydroxide ion will attach to the carbon by double bonds, wherein less than N.
On a similar mechanism and rule alkenes react chemically with molecules of the hydrogen halides.
Interest and the reaction of polymerization, which formed high-molecular compounds. So, selecting as the initial monomer of ethylene catalytic polymerization produces polyethylene, which is a valuable raw material for chemical industry.

Physical properties
The First members of the homologous series of ethylene are gaseous substances are practically odorless. They are poorly soluble in water, soluble in organic compounds. There is a direct correlation between the increase in relative molecular mass and boiling points (melting point), transition from gaseous state to liquid, solid state of aggregation.
Conclusion
Where usedrepresentatives of the unsaturated hydrocarbons of the ethylene series? Ethylene is a valuable feedstock for chemical production. It is possible to obtain styrene, vinyl chloride, ethanol, acetic aldehyde, acid, and dichloroethane. In the polymerization of alkenes formed polyvinyl acetate, lubricants, rubbers. On average, global production of polyethylene is 100 million tons per year. Industrial quantities of propylene required for the synthesis of the polymer. In addition, the propene is the raw material for the manufacture of oxide, isopropanol, cumene, butyric aldehyde, glycerine.
Butyny basically needed to create a polyisobutylene, methyl ethyl ketone, butyl rubber, isoprene. Isobutylene is an excellent chemical feedstock in the manufacture of tertiary butanol, butyl rubber, and isoprene. It is the isobutylene necessary for alkylation of phenols in the manufacture of surfactants. Copolymers with butene used as a sealant and oil additives. Higher unsaturated hydrocarbons of the ethylene series are used not only in the production of polymeric materials, but also organic production of higher alcohols.
Article in other languages:
AR: https://tostpost.com/ar/education/2210-alkenes-alkenes.html
HI: https://tostpost.com/hi/education/2211-alkenes-alkenes.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
the Telegram That the word may conjure up the image of a yellowed tattered paper containing the message about some distant historical events that are not closely related to the modern world. However, many methods currently in use ...
Skagen is the place where the borders of the Baltic sea and the North sea
we Invite you to enjoy a cozy resort town, the most Northern point of Denmark. In addition, Skagen — a city of great scenic beauty, you can witness a unique natural phenomenon. Here border the North and Baltic sea. Border wh...
"Matilda" - infantry support tank
the Original purpose of the armored forces is to suppress the resistance of enemy infantry and bridging lines of fortifications. The second world war overturned the established notions about the rules of warfare and tactics. Frequ...
The main natural areas of France and their characteristics
One of the most amazing countries in Europe is France. It is located between the Mediterranean sea and the Atlantic ocean. Due to this climate there is extremely PRIETEN and ideal for tourism. Now we will look closer at natural ar...
Mastodon is an ancestor of the elephant?
for anybody not a secret that in the ancient world was inhabited by unique animals, which, unfortunately or fortunately, we never got to see. Massive and huge but the remains indicate the Majesty and power of these mammals. So, in...
Types of worms: description, structure, their role in nature
There are three main types of worms: Flat, Round and Ringed. Each of them is divided into classes, in which are combined the types of worms by similarity of certain features. In this article we will describe types and classes. We ...






















Comments (0)
This article has no comment, be the first!