Thermodynamics is a... the Definition, laws, application and processes
What is thermodynamics? It is the branch of physics that deals with the study of the properties of macroscopic systems. This study also directed to methods of energy conversion and methods of its transmission. Thermodynamics is the branch of physics that studies the processes occurring in the system and their status. That still falls into the list of studied her things today and we'll talk.
Definition
In the picture below you can see an example of the images obtained in the study of the jug with hot water.
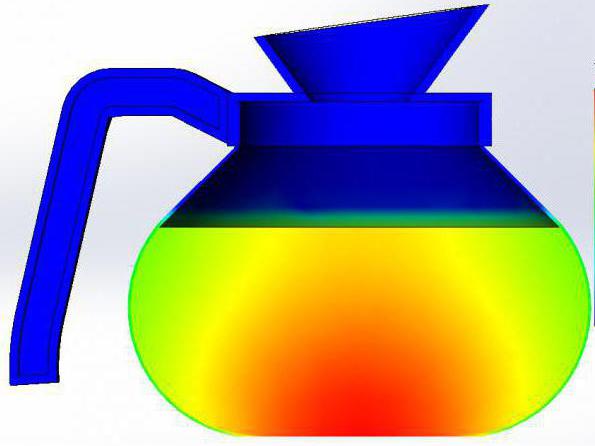
Thermodynamics is the science, which is based on generalized facts obtained empirically. Occurring in thermodynamic systems, the processes are described by using the macroscopic quantities. Their list includes such parameters as concentration, pressure, temperature and the like. It is clear that the individual molecules they do not apply, and are reduced to the description of the system in its General form (as opposed to those quantities used in electrodynamics, for example).
Thermodynamics is the branch of physics that also has its own laws. They, like the rest of them are of a General nature. Specific structural details of one or another of the chosen substance will not have a significant impact on the character of laws. That is why I say that this branch of physics is one of the most applicable (or, rather, is successfully applicable) in science and technology.
Usage

To List the examples can be very long. For example, many solutions based on thermodynamic laws, can be found in the field of thermal equipment and electric power industry. What to say about the description and understanding of chemical reactions, phase transitions, transport phenomena. In some ways, thermodynamics "cooperating" with the quantum dynamics. The area of contact is the description of the phenomenon of black holes.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Laws
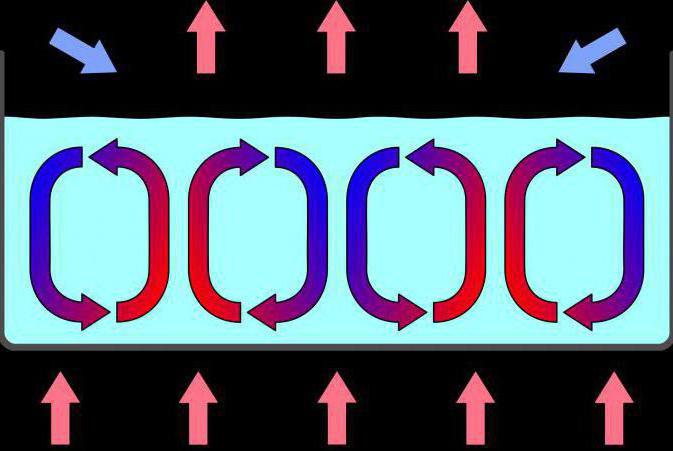
The Picture above demonstrates the essence of one of the thermodynamic processes - convection. Warm layers of substance rise up, cool - down.
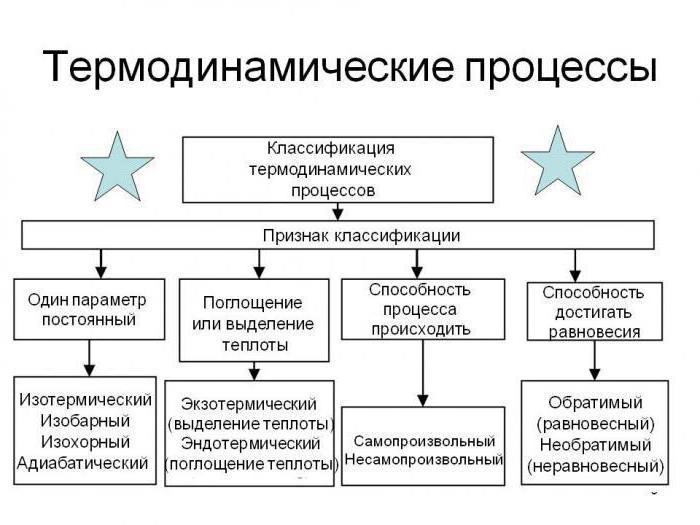
An Alternative name of laws, which, incidentally, is not used in the example often, it is a law of thermodynamics. Today there are three (plus one "zero", or “General”). But before I talk about that involves each of the laws will try to answer the question of what law of thermodynamics.
They represent a set of certain postulates that form the basis for understanding the macro-systems processes. The provisions of the beginnings of thermodynamics were established empirically as of the entire series of experiments and research. Thus, there is some evidence that allows us to take the postulates adopted without a single doubt in their accuracy.
Some people question about why thermodynamics need these laws. Well, we can say that the need for their use due to the fact that in this section the physics of macroscopic parameters are described in a General way, without any hint of consideration of their microscopic nature, or characteristics of the same plan. This is the sphere not of thermodynamics and statistical physics, already specifically. Another important thing is the fact that the law of thermodynamics are independent from each other. That is one of the second display will not work.
Usage
Application of thermodynamics, as mentioned earlier, it is in many areas. The basis is, incidentally, one of started it, which otherwise is interpreted in the form of the law of conservation of energy. Thermodynamic solutions and postulates are successfully implemented in industries such as energy, Biomedicine, chemistry. Here in biological energy is used throughout the law of conservation of energy and the law of probability and the direction of thermodynamic process. Along with this, there are three most common concepts that underpin all of the work and its description. This thermodynamic system, process and phase.
Processes
Processes in thermodynamics have a different degree of difficulty. There are seven pieces. Generally, under the process in this case should be understood not that other, as the change in the macroscopic state in which the system was given earlier. It should be understood that the difference between the conditional initial state and the end result can be negligible.
If the difference is infinitesimal, the incident process, we may call elementary. If we are going to discuss, we will have to resort to the mention of additional terms. One of them is "working body". Working body is a system in which there is a heating process or more.
Conventionally, the processes are divided into non-equilibrium and equilibrium. In the case of the latter all the States through which will undergo a thermodynamic system, are respectively non-equilibrium. It often happens that a change state is in such cases rapidly. But the equilibrium is close to quasi-static processes. Changes are order of magnitude slower.
Thermal processes in thermodynamic systems, can be reversible and irreversible. In orderto understand, let's break in its submission the sequence of actions at certain intervals. If we can do the same process in the opposite direction with the same “intermediate stations”, it can be called reversible. Otherwise make it not work.
Article in other languages:
PT: https://tostpost.com/pt/educa-o/9964-a-termodin-mica-a-defini-o-leis-aplica-o-e-processos.html
TR: https://tostpost.com/tr/e-itim/9976-termodinamik---bu-tan-m-yasalar-uygulama-ve-s-re-leri.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Novokuznetsk - what area? Novokuznetsk on the map of Russia
Many in the Russian Federation cities whose fame has spread far beyond the country's borders. One of these settlements is Novokuznetsk.General informationRussia, Kemerovo region, Novokuznetsk – the exact address of the locat...
How to formulate the problem of text?
the last task of the exam on the Russian language from the student is required to read the text and find the problem. Those in job, there are three, sometimes more. Problems with their definition, occur infrequently. However, it i...
Twin cities. What this means in the modern world?
In the Soviet ideology, except for an exaggerated patriotism, a bigger role for the thesis of the friendship between the peoples of different countries and continents. This picture is very smoothly blended with the phenomenon of s...
the Theory of the elites in the classical form belongs to such figures as Pareto, Mosca, Michels. They initiated the development of subsequent concepts. The modern theory of the elites is quite diverse. Among them there are a numb...
How to draw an octopus - step by step lesson
In this article we will discuss how to draw an octopus. Anyone who enjoys painting or is just starting this lesson will be very useful.Octopus is not as cute as puppies and kittens, but this is a very interesting sea inhabitants. ...
What is a household? The functions of the household. The household
It is the state of the household directly affects the well-being of the population and its material well-being. Currently, the rural household is a massive economic entity, it draws the attention of the government, many small farm...






















Comments (0)
This article has no comment, be the first!