The second law of thermodynamics: definition, meaning, history
Thermodynamics as an independent part of physical science appeared in the first half of the nineteenth century. Came the machine age. The industrial revolution demanded to study and to understand the processes related to the functioning of heat engines. At the dawn of the machine age inventors-singles could afford to use only intuition and "at random". There were no public order on discoveries and inventions, no one even could not imagine that they can be useful. But when the heat (and later electric) machines have become the basis of production, the situation has changed. Scientists finally gradually dealt with terminological confusion that prevailed until the mid-nineteenth century, to determine what is called energy, that force, that impulse.
What postulates thermodynamics
Let's Start with common knowledge. Classical thermodynamics is based on several postulates (principles), successively introduced in the course of the nineteenth century. That is, these provisions are not provable within it. They were formulated as a result of generalization of empirical data.
The First – this application of the law of conservation of energy to describe the behavior of macroscopic systems (consisting of a large number of particles). Briefly it can be formulated as: the stock of internal energy of an isolated thermodynamic system always remains constant.
The meaning of the second law of thermodynamics is to determine the direction in which processes occur in such systems.
The beginning of the Third allows you to accurately determine such value as entropy. Will consider it in more detail.
The Concept of entropy
The formulation of the second law of thermodynamics was proposed in 1850 by Rudolf Clausius: “Impossible spontaneous transfer of heat from the less heated body to more heated one”. The Clausius emphasized the merit of Sadi Carnot in 1824, who established that the proportion of energy that can be converted into work heat engine depends only on the temperature difference between the heater and the refrigerator.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...

In further elaboration of the second law of thermodynamics Clausius introduces the concept entropy - measures the amount of energy that is irreversibly transformed into a form, for the treatment to work. Clausius expressed this value by the formula dS = dQ/T, where dS, which determines the entropy change. Here:
DQ - change in heat;
T – absolute temperature (the one which is measured in Kelvins).
A Simple example: touch the hood of your car with the engine running. It is clearly warmer than the surrounding environment. But the car engine is not designed to heat the hood or the water in the radiator. Converting the chemical energy of gasoline into heat and then into mechanical, he's doing useful work – rotates the shaft. But a large part of the generated heat is lost, since no useful work to remove him from the impossible, and that flies out the exhaust, there's no way the gasoline is not. The thermal energy is lost, but does not disappear, but is dissipated (dissipare). Hot hood, of course, cools down, and each cycle of the cylinders in the engine again adds to his warmth. Thus the system tends to reach the thermodynamic equilibrium.
Entropy
Clausius deduced a General principle for the second law of thermodynamics in the formula dS ≥ 0. Physical sense it can be defined as "increasing" entropy: in a reversible process, it is not changed in irreversible increases.
It Should be noted that all real processes are irreversible. The term "increasing" reflects the fact that in the consideration of the phenomena included also theoretically possible ideal scenario. That is, the amount of unavailable energy in any spontaneous process increases.
The Possibility of reaching absolute zero
Max Planck has made a major contribution to the development of thermodynamics. In addition to working on the statistical interpretation of the second principle, he took an active part in the postulation of the third law of thermodynamics. The first formulation belongs to Walter Nernst and dates back to 1906. Wave equation considering the equilibrium behavior of the system at the temperature tending to absolute zero. First and second law of thermodynamics do not allow to figure out what will be the entropy in these conditions.

At T = 0 K energy is zero, the particle system will stop the chaotic thermal motion and form an ordered structure, a crystal with thermodynamic probability equal to one. Hence, the entropy also goes to zero (below we find out why this is so). In reality, it even makes it a little bit earlier, which means that the cooling of any thermodynamic system, of any body to absolute zero is impossible. The temperature is arbitrarily close to that point but never reach it.
Perpetuum-mobile: it is impossible, even if you really want
Clausius summarized and formulated the first and second law of thermodynamics thus: the total energy of any closed system always remains constant, and the total entropy increases over time.
The First part of this statement prohibits perpetual motion machine of the first kind – a device that performs work without the influx of energy from an external source. Secondpart forbids a perpetual motion machine of the second kind. Such a machine would transfer the energy of the system in operation without entropy compensation without violating the conservation law. It would be possible to pump heat out of equilibrium systems, for example, to fry an egg or pouring the steel due to the energy of thermal motion of water molecules, cooling it at the same time.
The Second and third law of thermodynamics forbid perpetual motion machine of the second kind.
Alas, nature has nothing to do not only for nothing, and still have Commission to pay.
“Heat death”
There are Few scientific concepts that have caused so much mixed emotions, not only among the General public, but among scientists themselves, how many fell on the share of entropy. Physics, first and foremost, the Clausius, almost immediately extrapolated the law of non-decreasing, first to Earth and then the Universe (and why not, after all, it is also possible to consider the thermodynamic system). In the end, the physical quantity, an important element calculations in many technical applications, was perceived as the embodiment of a universal Evil, destroying the light and the good in the world.
Among scientists there are such opinions: since, according to the second law of thermodynamics, entropy irreversibly increases, sooner or later, all the energy of the Universe is degraded to the scattered form, and will come "heat death". What's to celebrate? Clausius, for example, a few years did not dare to publish their findings. Of course, the hypothesis "heat death" immediately caused many objections. Serious doubts as to its correctness is now.
Demon-sorter
In 1867, James Maxwell, one of the authors of the molecular-kinetic theory of gases, in a very clear (albeit fictional) experiment demonstrated the apparent paradox of the second law of thermodynamics. Brief experience can be summarized as follows.
Suppose you have a container of gas. The molecules in it move chaotically, their rate differ slightly, but the average kinetic energy is the same throughout the vessel. Now divide the vessel with a partition into two isolated parts. Average speed of molecules in both halves of the vessel will remain the same. Partition guarded by a tiny demon that allows faster, “hot” molecules to penetrate one part, and a slower “cold” in another. As a result, in the first half, the gas will heat up in the second – will be cooled, that is, from the state of thermodynamic equilibrium the system will move to the difference of thermal capacities, which means a decrease of entropy.
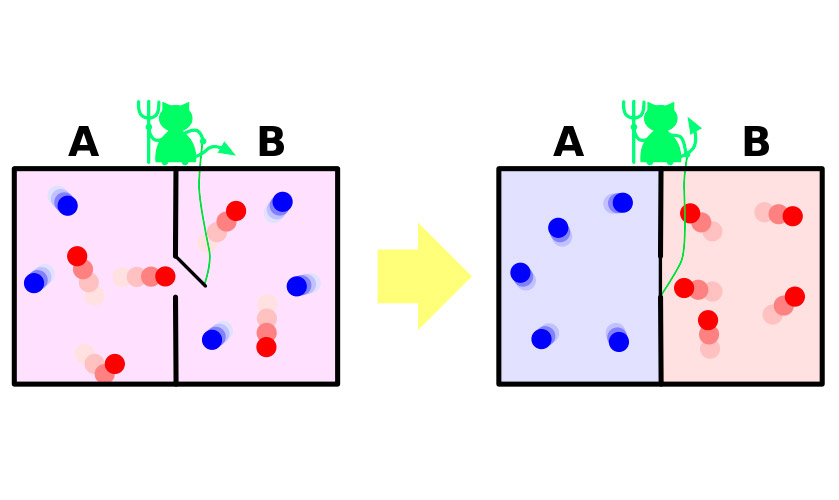
The problem is that in the experiment system to make this transition spontaneously. It receives outside energy which opens and closes a partition or system by necessity includes a demon, spending your energy on the duties of gatekeeper. The increase in entropy of the demon abundantly cover the reduction in her gas.
Undisciplined molecules
Take a glass of water and leave it on the table. Watch the glass is not necessarily enough some time to come back and check on the status of water in it. We will see that its amount is decreased. If you leave the glass for a long time, it is generally not found water, as all of it will evaporate. At the beginning of the process, all water molecules were located in a restricted walls of the glass region of space. At the end of the experiment, they were scattered across the room. The volume of the molecules have much more opportunities to change their location without affecting the state of the system. We have no way to collect them soldered "team" and put back into the glass, with the health benefits of drinking water.
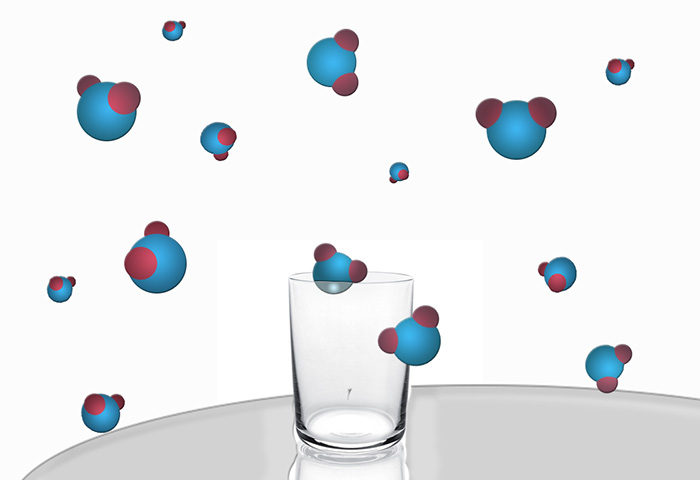
This means that the system evolved towards a state with higher entropy. On the basis of the second law of thermodynamics, entropy, or the process of dispersion of particles in the system (in this case water molecules) are irreversible. Why is it so?
Clausius did not answer this question or anyone else couldn't do that to Ludwig Boltzmann.
Macro and microsetae
In 1872, this scientist introduced the scientific statistical interpretation of the second law of thermodynamics. Because a macroscopic system, with whom it has thermodynamics, established a large number of elements whose behavior is subject to statistical laws.
Return to the water molecules. Randomly flying across the room, they can occupy different positions, have some difference in speeds (molecules constantly collide with each other and with other particles in the air). Each state of the system of molecules is called a microstate, and there are a huge number of options. In the implementation of the vast majority of options macrostate of the system will not change in any way.
Nothing is forbidden, but something extremely unlikely
The Famous ratio S = k lnW associates a number of possible ways that you can Express a certain macrostate of a thermodynamic system (W), its entropy S. the Value of W called the thermodynamic probability. The final form of this formula gave Max Planck.K-factor – an extremely small amount (of 1.38×10−23 j/K), characterizing the relationship between energy and temperature, Planck named the Boltzmann constant after the scientist who first proposed the statistical interpretation of the second law of thermodynamics.

It is Clear that W – always a natural number 1, 2, 3, ... N (there is no fractional number of ways). Then the logarithm of W, and hence entropy cannot be negative. When the only possible system the microstate entropy becomes zero. If you go back to our glass, this postulate can be represented as follows: the water molecules, randomly scurrying around the room, back into the glass. In addition, each exactly repeated their way and took the glass, same place, in what remained before departure. Nothing prohibits the implementation of this option, in which the entropy is zero. Only wait for the implementation of such a vanishingly low probability is not worth it. This is one example of what can be done only theoretically.
All mixed up in the building…
So, the molecules randomly fly around the room in different ways. There is no regularity in their arrangement, there is no order in the system, no matter how change the options of microstates, do not seem to be any coherent structure. In the glass was the same, but because of the limited space of the molecule changed its position not as active.
A Chaotic, disordered state of the system as the most likely corresponds to its maximum entropy. Water in a glass is an example of a more discountshopping state. The transition from uniformly distributed through the room of chaos impracticable.
Give more clear example for all of us - cleaning the mess in the house. To put everything in places, we also have to expend energy. In the process, it becomes hot (i.e., we do not freeze). It turns out that entropy can benefit. This is the case. You can say even more: entropy, and through it the second law of thermodynamics (along with energy) govern the Universe. Take a look at the reversible processes. So would the world not be of entropy: no development, no galaxies, stars, planets. No life.
Some More information about "heat death". There's good news. Since, according to statistical theory, “forbidden” processes are in fact unlikely, in thermodynamically equilibrium system fluctuations-spontaneous violation of the second law of thermodynamics. They can be arbitrarily large. With the inclusion of gravity in the thermodynamic system, the distribution of particles will not be randomly-uniform, and the state of maximum entropy will be reached. In addition, the universe is immutable, constant, stationary. Hence, the question of "heat death" sense.
Article in other languages:
KK: https://tostpost.com/kk/b-l-m/2269-termodinamikany-ek-nsh-bastamasy-any-tamasy-m-n-tarihy.html
TR: https://tostpost.com/tr/e-itim/2274-kinci-ba-lang-termodinami-in-tan-m-anlam-tarih-esi.html
UK: https://tostpost.com/uk/osv-ta/2271-druge-pochatok-termodinam-ki-viznachennya-zm-st-stor-ya.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
The Amundsen sea: Geology, climate, fauna
The North Pacific ocean on the one hand surround the glaciers, and on the other – West coast of Antarctica. The entire surface of pond covered with ancient ice.next to him cut into the permafrost, pointed toe Dart. To the Ea...
The Viscount is... the origin of the title
Medieval nobility, the hierarchy remains for many of our contemporaries are complex and confusing. This is not surprising, as even among his contemporaries noble families often were differences in the importance and reliability of...
Lieutenant-General Nikolay Vlasik, chief of the bodyguard "leader"
This man has lived a difficult life. It was challenging time itself. Lieutenant General Russia of today have a different reputation, however, without belittling the unqualified merits, the share of few of them have had something t...
Key dates in the history of Russia. Significant dates in the history of Russia
Russian Federation – a state that ranks first in area and ninth in population. It is a country which took the path from scattered principalities to the candidate for the superpower. How did the emergence of this political, e...
The Soviet government. The establishment of Soviet power
After the October revolution, the first Soviet power was established in most parts of the country. It happened in a fairly short period of time – March 1918 In most provincial and other large cities, the establishment of the...
Work plan the class teacher with a difficult child at school
Based on the fact that the family-unit of society, the formation of personality of each child primarily occurs in it. Children almost mindlessly copy the behavior of adults, not separating the wheat from the chaff. That is, the un...






















Comments (0)
This article has no comment, be the first!