Now - 21:47:34
What is the valence of oxygen in compounds?
To determine the possible values of the valences of oxygen, you should examine the position of the element in the periodic table, the main features of the structure of its atom. This approach is useful when studying the question of what is the valence of oxygen is typical and which is unusual for him. The most common connection appears normal valence — II. This feature allows you to define a number of connections other atom in the binary formulas, with the participation of oxygen.
What is the valence of oxygen?
At the initial stage of accumulation of knowledge about the properties and structure of substances chemists thought that valence is the ability to bind a certain number of atoms in a molecule of a substance. Many scientists after the discovery of the element was trying to understand what is the valence of oxygen. The response was obtained experimentally: the oxygen attaches in a chemical reaction two atoms of univalent hydrogen, therefore, bivalent. Ideas about the chemical bond were changed as knowledge of the structure of matter. In his theory of valency, Lewis and W. Kossel disclose the nature of the chemical interaction from the point of view of electronic structure. The researchers explained the ability of an atom to the formation of a certain number of links desire to the most stable energy state. If it is achieve the smallest particle of matter becomes more stable. In the theory and Lewis structures great attention is paid to the role of the outer electrons that take part in creating chemical bond.
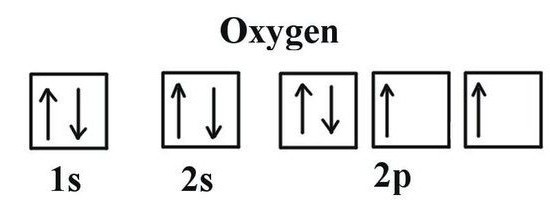
Features of distribution of oxygen in the periodic table
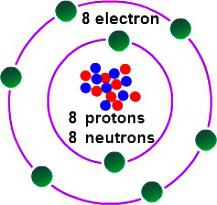
In order to determine the valence of oxygen, it is necessary to consider some peculiarities of its electronic structure. Oxygen leads the group 16 of the periodic table. Trivial family name of the elements — “the chalcogens”, according to the older classification they belong to VI(A) group. In the periodic table oxygen is in cell No. 8. The kernel contains in its structure 8 the same positive and neutral elementary particles. In the space of an atom has two energy levels that arise when moving 8 electrons, 6 of which — foreign.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...

What is the relationship between the composition of the atom and the valence?
On the last level of the oxygen atom contains 2 unpaired electrons. The element is inferior to the fluorine value of the electronegativity (ability to attract connecting the electronic pairs). In the formation of compounds with other elements oxygen attracts arisen in the molecule the total electron density (excluding electrons of fluorine). The achievement of steady state the outer shell is possible with the addition of two negative charges. This means that oxygen requires 2 electrons. You have the following options: accept one electron (valence II) to take other atom 2 electrons (valence II) to accept electrons from other atoms (valence 0). The typical behavior of oxygen characterizes the second case. This method can be used to find out what is the valence oxygen has the most typical in its common compounds. These include most oxides of metals and nonmetals.
How does valence in the compounds?
Oxygen is able to interact directly with many chemical elements. Known for its connections with practically all representatives of the periodic table (excluding inert gases: argon, helium, neon). In reaction with Halogens, noble metals, oxygen can not directly enter, but oxides Au2O3, F2O, Cl2O7 and others are (obtained indirectly). For binary compounds, the formation of which involved oxygen, typical covalent bond and polarity. Valency of molecules depends on the number of resulting pairs of electrons, which are attracted to the nucleus of different atoms. The vast majority of compounds the oxygen atoms are involved in covalent bonds. For example, oxides of CO2, P2O5, SO2, SO3, K2In2O3, Mo2O5 and other molecules. In the hydronium cation H3O+ oxygen is showing unusual for him valency III. The presence of pericarp –—– due to the unusual nature of hydrogen peroxide, H2O2. In this compound the oxygen manifests its inherent valence II.
How to determine the valence of elements?
The Idea of valency possibilities of oxygen gives a the structure of the Lewis — the chemical symbol of the element, around which the points noted by the electrons of the outer layer. They participate in the creation of molecules that are part of shared electron pairs. Formula Lewis demonstrates the valence of the oxygen, corresponding to the number of its unpaired electrons (2). The same result is the use of electronic graphic structures. In two cells in the outer energy level of oxygen located unpaired electrons (indicated in the formula by arrows). Information about what is the valence oxygen,allow you to define the finished formula of binary compounds the value for the neighboring atoms. To do this, carry out simple calculations. First, multiply the number of atoms On the conventional oxygen valence. The obtained value must be divided by the index that is specified in the formula next to the chemical symbol of another element in a compound with oxygen. With a simple way to calculate the valence of carbon and phosphorus into their oxides.
- Multiply the index at the bottom right of the sign On the dioxide CO2 to the typical valence of the element: 2 * 2 = 4. The number you divide the index specified for carbon: 4/1 = 4. Dioxide CO2 carbon is in its highest valence state IV.
- The index of the bottom right of the chemical symbol of oxygen in the phosphorus oxide R2O5 multiply by the typical valence of an atom is About: 5 * 2 = 10. This number divide by the specified in the formula index at the bottom right from the phosphorus atom: 10/2 = 5. The oxide of phosphorus is in its highest valence V.
Article in other languages:
AR: https://tostpost.com/ar/education/3230-what-is-the-valence-of-oxygen-in-compounds.html
BE: https://tostpost.com/be/adukacyya/5703-yakaya-valentnasc-k-slarodu-zluchennyah.html
DE: https://tostpost.com/de/bildung/5703-welche-wertigkeit-haben-sauerstoff-in-den-gelenken.html
ES: https://tostpost.com/es/la-educaci-n/5708-qu-valencia-el-ox-geno-en-las-conexiones.html
HI: https://tostpost.com/hi/education/3232-valence.html
JA: https://tostpost.com/ja/education/3231-what-is-the-valence-of-oxygen-in-compounds.html
KK: https://tostpost.com/kk/b-l-m/5705-anday-valentnost-u-otteg-osylystarda.html
PT: https://tostpost.com/pt/educa-o/5704-qual-a-val-ncia-perto-de-oxig-nio-nas-liga-es.html
TR: https://tostpost.com/tr/e-itim/5709-ne-de-erlik-atlayabilirsiniz-var-oksijen-bile-ikleri.html
UK: https://tostpost.com/uk/osv-ta/5707-yaka-valentn-st-u-kisnyu-v-spolukah.html
ZH: https://tostpost.com/zh/education/3479-what-is-the-valence-of-oxygen-in-compounds.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Battleship Nelson: the story of creation and service
Battleship ‘Nelson’ – the lead ship of the series, consisting of two units built for the Royal Navy after the First world war. The ship had a unique layout – the three towers of the main caliber was install...
Samara state Academy of culture and arts: description, faculties and reviews
Samara state Academy of art and culture – an educational organization that existed in Samara and worked in the field of higher education. It is now also operational, but under a slightly different name. The documents indicat...
What is the height of the ISS orbit? The orbit of the ISS around the Earth
the international space station was launched into space in 1998. Currently almost seven thousand days, day and night, the best minds are working on solving complex puzzles in zero gravity.Outer spaceEveryone, at least once saw thi...
Kiss of Mary Pickford: biography and photos
Perhaps no actress subsonic movie didn't have such popularity as Mary Pickford. Actress of theatre and cinema, the first business lady of Hollywood, the founder of a number of acting nominations and so on and so forth. It's hard t...
Odintsovo humanitarian University (OSU): student testimonials
Odintsovo, located in the Moscow region, previously existed OGU (Odintsovo University for the Humanities). It studied quite a lot of students. The University represents an Autonomous nonprofit educational organization, which is en...
Nuance is subtlety. The meaning, spelling, interpretation
In life there are many subtleties. Let this simple idea be the prologue to our conversation. And we are talking today about the nuances – as is well known, various parts.Significancedictionary – the thing is absolutely...















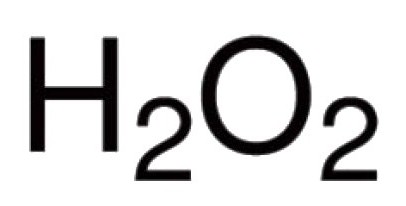






Comments (0)
This article has no comment, be the first!