Now - 20:56:40
What is adiabatic process?
To build a thermal machine that can perform work through the use of heat, it is necessary to create certain conditions. First of all, a heat engine must operate in a cyclic mode, where a series of sequential thermodynamic processes create a cycle. As a result of cycle gas enclosed in a cylinder with a movable piston, doing work. But one cycle for periodically operating the machine a little, it needs to execute cycles over and over again for a certain time. The total work performed within a specified time, in reality, divided by the time gives another important concept – power.
In the mid-nineteenth century were created the first thermal machine. They produced the work, but spent a large amount of heat produced by the combustion of fuel. It was then that theoretical physicists started wondering: “As the working gas in the thermal machine? How to get the maximum work with the minimum use of fuel?”
To perform the analysis of the work gas needed to introduce a system of definitions and concepts. The combination of all the definitions and created a whole new scientific field called: "engineering thermodynamics". In thermodynamics has been adopted by a number of assumptions, does not diminish the main conclusions. Working the body – ephemeral gas (not existing in nature), which can be compressed to zero volume, whose molecules do not interact. In nature there are only real gases that have distinct properties, distinct from an ideal gas.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
To consider the model of the dynamics of the working fluid was proposed the laws of thermodynamics, which describe basic thermodynamic processes, such as:
- Isochoric process – it is a process that occurs without change of volume of the working fluid. Condition isochoric process, v=const;
- Isobaric process – it is a process that occurs without a change in pressure in the working medium. The condition for Isobaric process P=const;
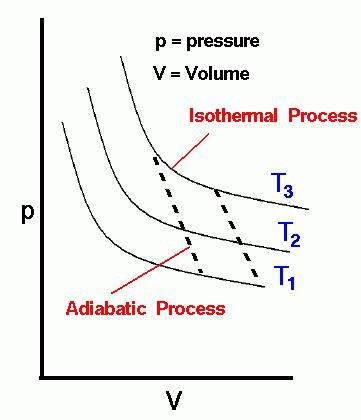
- Insulated (isothermal) process – it is a process that is performed while maintaining the temperature at a predetermined level. The condition of isothermal process, T=const;
- Adiabatic process(adiabatic, so-called modern heat) – a process occurring in space without exchange of heat with the environment. The condition for adiabatic process, q=0;
- Polytropic process – this is the most generalized process that describes all the above mentioned thermodynamic processes, as well as all other possible to commit in a cylinder with a movable piston.
During the creation of the first heat engines was looking for the loop, which can get very high efficiency (coefficient of performance). Sadi Carnot, explore the complex thermodynamic processes, on a whim came to developing its cycle, named after him – the Carnot cycle. It consistently performed isothermal, then adiabatic process of compression. The working fluid after the execution of these processes has energy inside, but the cycle is not complete, so the working fluid expands and performs isothermal expansion process. To complete the cycle and return to the original parameters of the working medium, in progress adiabatic process extensions.
Carnot proved that the efficiency in his cycle reaches its maximum and depends only on the temperatures of the two isotherms. The higher the difference between them is, consequently, higher thermal efficiency. Attempts to create a heat engine in the Carnot cycle did not succeed. It's a perfect cycle that cannot be performed. But he proved the main principle of the second law of thermodynamics about the impossibility of getting a job, equal to the cost of thermal energy. We formulated a number of definitions of the second beginning (the law) of thermodynamics based on which Rudolf Clausius introduced the concept of entropy. The main conclusion of his research – entropy is constantly increasing, which leads to the heat “death”.
The greatest achievement of Clausius was the understanding of the adiabatic process, when executed, the entropy of the working fluid does not change. So adiabatic process the Clausius – it is s=const. Here s-is the entropy, which gives another name to the process carried out without supply or removal of heat, - isentropic process. Scientist engaged in search of such a cycle heat engine, where there would be an increase in entropy. But, unfortunately, he failed to create. Therefore, deduced that a heat engine cannot be created at all.
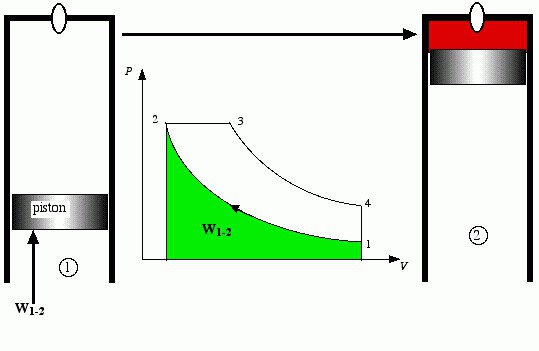
But not all researchers have been configured so pessimistic. They were looking for the real cycles of heat engines. As a result of their searches Nikolaus August Otto created his own cycle heat engine, which is now being implemented in engines running on gasoline. Here are adiabatic process of compression of the working body and isochoric heat input (combustion at constant volume), then there adiabata expansion (work is done body work in the process of increasing its volume) and isochoric heat removal. The first internal combustion engines according to the Otto cycle is used as fuel flammable gases. Much later they invented the carburetor, which began to create air-petrol mixtures of air with vapors of gasoline and deliver it to the cylinderengine.
Otto cycle is compressed combustible mixture, so the amount of compression of its relatively small – a combustible mixture tends to detonate (explode upon reaching a critical pressure and temperature). Therefore, the work in the adiabatic process of compression is relatively small. Here is introduced another concept: the degree of compression-the ratio of the total volume to compress.
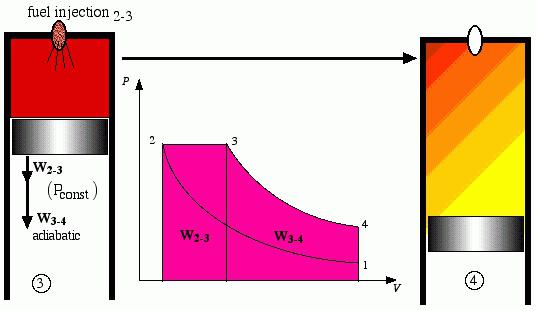
Finding ways of increasing the efficiency of energy use and fuel continued. The increase in efficiency was seen to increase the compression ratio. Rudolf Diesel developed the cycle in which supply of heat is carried out at constant pressure (Isobaric process). Its cycle was the basis of engines that use diesel fuel (sometimes called diesel oil). In the cycle of a Diesel engine is compressed fuel mixture, and air. So I say, what is done work in the adiabatic process. The temperature and pressure at end of compression is high, so using the injector is fuel injection. It is mixed with the hot air forms a combustible mixture. It burns, it increases the internal energy of the working fluid. Further, the gas expansion is on adiabate, committed stroke.
An Attempt to realize a Diesel cycle in heat engines failed, so Gustav Trinkler created combined cycle Trinkler. It is used in today's diesel engines. In the cycle of Trinkler heat is supplied by isochore and then isobare. Only after this is done adiabatic process expansion of the working body.
By analogy with piston heat machine work and turbine. But the process of removal of heat at the end of the useful of the adiabatic expansion of the gas is performed by isobare. On aircraft with gas turbine and turboprop engines adiabatic process is performed twice during the compression and expansion.
To justify all the basic concepts of adiabatic process, was proposed formulas. Here appears an important quantity, called the ratio of specific heats. The value for diatomic gas (oxygen and nitrogen – these are the basic diatomic gases present in the air) is equal to 1.4. To calculate the ratio of specific heats are used to two interesting characteristics, namely, the Isobaric and isochoric heat capacity of the working fluid. The ratio of their k=Cp/Cv – is the ratio of specific heats.
Why in the theoretical cycles of heat engines used an adiabatic process? Actually are polytropic processes, but due to the fact that they occur at high speed, it is customary to assume the absence of heat exchange with the environment.
90% of electricity is produced in thermal power plants. As the working fluid is used water vapor. It is obtained by boiling water. To increase the capacity of the steam, it is superheated. Then high pressure superheated steam is supplied to steam turbine. Here also occurs adiabatic process of expansion. Turbine is actuated, it is passed to the generator. That, in turn, generates electricity for consumers. Steam turbines operate on the Rankine cycle. Ideally, the improvement of efficiency is also associated with an increase in temperature and vapour pressure.
As seen from above, the adiabatic process is very common in the production of mechanical and electrical energies.
Article in other languages:
AR: https://tostpost.com/ar/education/16531-what-is-adiabatic-process.html
BE: https://tostpost.com/be/adukacyya/32060-shto-takoe-adiabaticheskiy-praces.html
DE: https://tostpost.com/de/bildung/31736-was-ist-der-adiabatische-prozess.html
ES: https://tostpost.com/es/la-educaci-n/31598-qu-es-adiabaticheskiy-el-proceso.html
HI: https://tostpost.com/hi/education/18173-adiabatic.html
JA: https://tostpost.com/ja/education/16229-what-is-adiabatic-process.html
KK: https://tostpost.com/kk/b-l-m/32388-b-l-adiabaticheskiy.html
PL: https://tostpost.com/pl/edukacja/33443-co-to-jest-adiabaticheskiy-proces.html
PT: https://tostpost.com/pt/educa-o/33193-o-que-adiabaticheskiy-processo.html
TR: https://tostpost.com/tr/e-itim/28804-nedir-adiabaticheskiy-s-reci.html
UK: https://tostpost.com/uk/osv-ta/32597-scho-take-ad-abatniy-proces.html
ZH: https://tostpost.com/zh/education/11006-what-is-adiabatic-process.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Maritime climate: definition, features, scope. Than the marine climate differs from continental?
Maritime climate or oceanic, – the climate of regions located near the sea. It is characterized by small diurnal and annual temperature changes, high humidity and atmospheric precipitation, falling in large quantity. Also ch...
Things to do on vacation: the best ideas
the School and the lessons left behind – the long-awaited break in an endless series of school days and homework. Often guys in advance thinking about what to do on vacation. Maybe someone is planning trips with friends or t...
History Kesem Sultan - brilliant life brilliant woman
History Kesem Sultan amazingly combines dense historical fabric with a subtle touch of fiction. Historians studying the customs and Chronicles of the Ottoman Empire, have different opinions about her impact on the Sultan. However,...
In the early 20th century in Russia, the main stood the agrarian question. At that time, the tsarist government followed economic policies that were aimed largely at maintaining the nobility of land are in decline. The government ...
Ecosystem called the special unity of plants, microorganisms and animals, in which between them there is an exchange of various substances and energy. Each ecosystem has its own soil composition, temperature, and other metrics. Th...
Danish physicist Niels Bohr: biography, open
Niels Bohr-Danish physicist and public figure, one of the founders of physics in its modern form. Was the founder and Director of the Copenhagen Institute for theoretical physics, the Creator of the world's scientific schools, as ...





















Comments (0)
This article has no comment, be the first!