Now - 00:49:31
The properties of electrolytes. Strong and weak electrolytes. Electrolytes - what is it?
Excellent conductors of electric current — gold, copper, iron, aluminum, alloys. In addition, there is a large group of substances-metals, melts and aqueous solutions which also have the property of conductivity. It is a strong bases, acids, some salts, generally referred to as "electrolytes". What is ionic conductivity? Find out what substances are electrolytes this common phenomenon.

What particles carry the charges?
The World around is full of different conductors and insulators. About these properties of bodies and substances known from antiquity. The Greek mathematician Thales had experience with amber (in Greek — “Elektron"). Rubbing it on silk, a scientist observed the phenomenon of attraction of hair and wool fibres. Later it became known that amber is an insulator. In this matter there is no particles that could carry an electric charge. Good conductors — metals. In their composition are the atoms, positive ions and free, negative infinitely small particles-the electrons. They provide a charge transfer when the current is passed. Strong electrolytes in dry form do not contain free particles. But when dissolving and melting is the destruction of the crystal lattice and the polarization of covalent bonds.
Water, non-electrolytes and electrolytes. What is dissolution?
Giving or attaching electrons, the atoms of metallic and nonmetallic elements become ions. Between them in the crystal lattice there is a fairly strong relationship. Dissolving or melting an ionic compounds, e.g. sodium chloride, leads to its destruction. In polar molecules, there is neither associated nor free ions, they occur when interaction with water. In the 30-ies of the XIX century, Michael Faraday discovered that solutions of some substances conduct electricity. The scientist introduced to the science of such crucial concepts:
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
- Ions (charged particles);
- Electrolytes (conductors of the second kind);
- The cathode;
- The anode.
There is a connection – strong electrolytes, crystal lattice which completely destroyed with the release of ions.
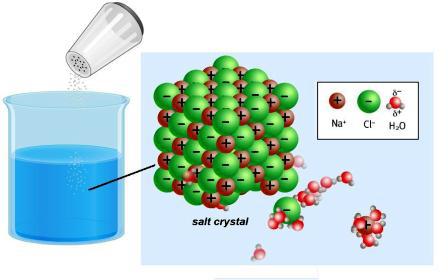
There are insoluble substances and those that remain in molecular form, e.g., sugar, formaldehyde. Such compounds are called non-electrolytes. They are not characterized by the formation of charged particles. Weak electrolytes (coal and acetic acid, ammonium hydroxide and other substances) contain few ions.
The Theory of electrolytic dissociation
In his works of the Swedish scientist S. Arrhenius (1859–1927) was based on the findings of Faraday. To further elaborate on elements of his theory by the Russian researchers I. Heels and V. Kistiakowsky. They found that the dissolution and melting to form ions is not all substances, but only electrolytes. What is dissociation by s. the specific rate constant? This is the destruction of molecules, which leads to charged particles in solutions and melts. The basic theoretical principles of Arrhenius, S.:
- Bases, acids and salts in solution are dissociated form.
- Reversible dissociate into ions strong electrolytes.
- Weak and form few ions.

Indicator of the degree of dissociation of the substance (often expressed as a percentage) is the ratio of the number of molecules that split into ions, and the total number of particles in the solution. Electrolytes are strong if the value of this indicator over 30%, from the weak — less than 3%.
Properties of electrolytes
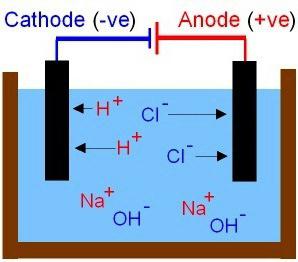
The Theoretical conclusions of Arrhenius S. complements more recent studies of physicochemical processes in solutions and melts was conducted by Russian scientists. Received an explanation of the properties of bases and acids. The former include compounds in solutions of cations which can be detected only metal ions, anions, are particles the OH–. Acid molecules break into negative ions and the acid residue and the hydrogen protons (H+). The movement of ions in solution and the melt — chaotic. Consider the results of the experience for which you'll need to make a chain to include carbon electrodes and ordinary incandescent bulb. Check the conductivity of solutions of different substances: salt, acetic acid and sugar (the first two – electrolytes). What is electric circuit? It is a current source and conductors connected between them. On closing the circuit the bulb will burn brighter in a solution of salt. The movement of ions becomes the order. The anions are directed to the positive electrode, and cations — negative.
In this process, acetic acid is involved a small number of charged particles. Sugar is not an electrolyte does not conduct current. Between the electrodes in this solution would be an insulating layer, the light bulb will not illuminate.
Chemical interactions between electrolytes
When merging solutions it is possible to observe the behavior of electrolytes. What are the ionic equations of these reactions? Consider the example of the chemical interaction between barium chloride and sodium nitrate:
2NaNO3 + BaCl2 + = 2NaCl + Ba(NO3)2.
Formula of electrolytes are written in ionic form:
2Na+ + 2NO3– + Ba2+ + 2Cl– = 2Na+ +2Cl– + Ba2+ + 2NO3–.
Taken for the reaction substance is a strong electrolyte. In this case, the composition of ions is not changed. Chemical interaction between the electrolyte solutions is possible in three cases:
1. If one product is an insoluble substance.
Molecular equation: Na2SO4 + BaCl2 = BaSO4 + 2NaCl.
Write a composition of electrolytes in the form of ions:
2Na+ + SO42– + Ba2+ + 2Cl– = BaSO4(white precipitate) + 2Na+ 2Cl–.
2. One of the resulting substances — gas.
3. Among the reaction products is a weak electrolyte.
Water — one of the most weak electrolytes
Chemically pure water (distilled) does not conduct electricity. But in its composition there are a small number of charged particles. It is the protons of N+ and anions of the HE–. Dissociation is exposed to a negligible number of water molecules. There is a variable — the ionic product of water, which is constant at the temperature of 25 °C. It allows to know the concentration of H+ and HE–. Is dominated by hydrogen ions in acid solutions, hydroxide anions, more in alkalies. In neutral — the same as the number of N+ and HE–. Environment solutions are also characterized by hydrogen index (pH). The higher it is, the more there is a hydroxide ion. Environment is neutral in the pH range close to the 6–7. In the presence of ions H+ and HE– change color of substances-indicators: litmus, phenolphthalein methylorange and others.
Properties of solutions and melts of electrolytes are widely used in industry, science, agriculture and medicine. Scientific justification inherent in the works of several eminent scientists, to explain the behavior of particles that make up salts, acids and bases. In their solutions occur various reactions of ion exchange. They are used in many industrial processes, in electrochemistry, electroplating. Processes in living beings also occur between ions in solutions. Many nonmetals and metals, are toxic in the form of atoms and molecules, essential in the form of charged particles (sodium, potassium, magnesium, chlorine, phosphorus, etc.).
Article in other languages:

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
The forager is a person, engaged in collection of fodder
"the Forager" is a very ancient word that can be used in different values.Now it can already be considered archaic - a relic of the past. However, it is often found in history books or manuals on animal husbandry. Who are foragers...
How to write an essay in English? Plan, structure and sample essays
Very often the final work during the verification of knowledge of English is writing essays. Many students don't like it because their level of language proficiency is not high enough. The reason lies in the fact that to write coh...
Bronze is an alloy. Characteristics of bronze
Bronze is an alloy based on copper. The auxiliary metal may be Nickel, zinc, tin, aluminum and others. In this article we consider the types, technological characteristics, chem. the composition of bronze, and the methods of its m...
How to calculate the volume of right geometric solids
throughout our lives we have to calculate the volume of certain geometric shapes. For example, during construction it is necessary to correctly calculate the amount of trenches and excavations. In addition, this value is determine...
the Task of referees is to thoroughly monitor compliance with the rules of the game, rules of competition and to be objective in determining the winner. Their entire lineup involved in service specific event, United for a judgeshi...
Glaciology - the science of what? That study glaciologist?
Glaciology – is the science about? What do the professionals that work in this field? Let's try to find the answer to these and other questions.Glaciology what is studying?the Term comes from the Latin word ‘glacies&rd...






















Comments (0)
This article has no comment, be the first!