Now - 04:03:01
Arsenic acid: chemical properties, formula. Highly hazardous substances
The Effects of different chemicals on the human body ambiguously. Most of us known compounds or are neutral or positive role in human life. But there is a group of substances, which pose a serious threat to health. They are divided into several classes. Discussed in this article arsenic acid – one such toxic chemical compounds. According to the currently accepted classification, it is included in the second class of danger, along with chloroform, lead compounds and lithium. Inspect the properties of arsenic acid in more detail.
The structure of the molecules and state of matter
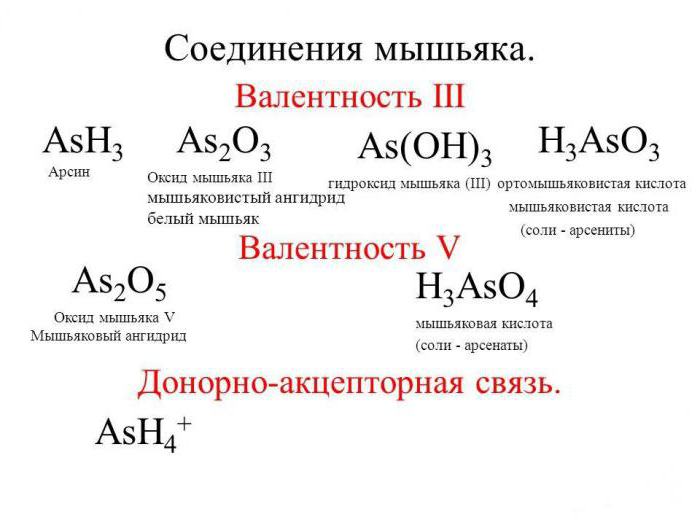
This connection under normal conditions has a crystalline structure. Being tribasic, arsenic acid, the formula for which is H3AsO4, has both medium and sour salt. For example, which of the following potassium-K2HAsO4, dihydroorotate sodium – NaH2AsO4, lithium arsenate-Li3AsO4. Prokaeva arsenic acid, get hemipenes of arsenic called arsenic anhydride. Its white clear crystals to form a glassy mass that is poorly soluble in water.
Dissociation
H3AsO4, along with formic acid and hydroxide of lead, is a moderately weak electrolyte. So, in the table of ionization of important acids ortesisacomo acid has three dissociation constants: 5.6 x 10-3 and 1.5 x 10-7 and 3, 89 x 10-12. These indicators quantitatively describe the acid strength. In accordance with the dissociation constants among inorganic acids H3AsO4 is a position between the chrome and antimony acids. Russian chemists-experimenters, A. L. and I. L. Agafonovy formulated a mathematical expression which brought the dependence of the first and second dissociation constants of arsenic acid from a temperature in the range from 0°C to 50°C.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Chemical properties
The Degree of oxidation of an atom of arsenic that is included in the molecule of the acid, equal to +5. This speaks to the fact that the connection itself in chemical reactions with other substances showing oxidative properties. So, when she interacts with the potassium iodide acting as a reducing agent, in an acidic medium among the products of the reaction we find arsenious acid H3AsO3. Recall that the arsenic acid, the formula for which is H3AsO4, Is tribasic, and hence in the reactions with alkalis or insoluble bases it can give three kinds of salts: average, hydro and dihydroorotate. Qualitative reaction on the ion AsO43- in analytical chemistry is the interaction of most arsenic acid or its salts with soluble silver salts, e.g. nitrate. The result is the observed deposition of Ag3AsO4 Coffee color.
Iodometric method for the determination of arsenic acid
In analytical chemistry, an important task is the detection of chemical compounds in the studied solutions. Arsenic acid chemical properties which we discussed earlier, can be detected by micromethod of iodometry. To 1 ml of its solution poured the same volume of 4n. hydrochloric acid solution and 1 ml of 4% solution of potassium iodide. Formed sesquioxide arsenic As2O3, Mass of which in strict observance of the quantitative amounts of the reactants are always the same and equal 0,5746 mg.

Oxidative capacity of arsenic acid
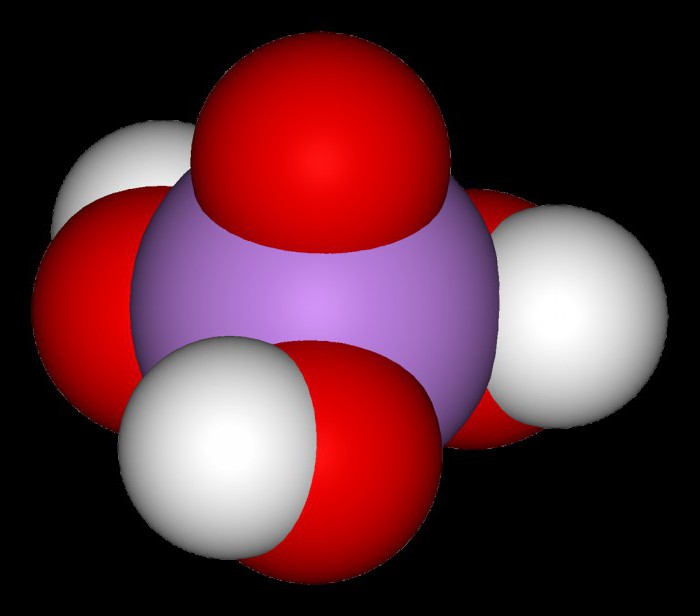
As we know, H3AsO4, As orthophosphoric acid, is a medium-strength electrolyte. Her white transparent crystals spread out in the air and have the compound 2H3AsO4 H2O. It of the salt formed with alkaline metals (such as medium-sized and acidic) in water solutions have a pH greater than 7. Arsenate lithium, potassium, sodium and ammonium are water soluble, and the rest are average salt will not dissolve. Arsenic acid – a good oxidizing agent. In redox reactions it is restored to arsenious acid or arsine.
H3AsO4 + 2e + 2H+ = H3AsO3 + H2O
H3AsO4 + 8e + 8H+ = AsH3 + 4H2O
In addition, the arsenic acid readily oxidizes various metals, sulfite and jodido acid, and hydrogen sulfide.
Obtaining arsenic acid
In the laboratory H3AsO4 Can be obtained by the reaction of sesquioxide of arsenic with nitric acid when heated. The products detected by the oxide of trivalent nitrogen and H3AsO4. Another way to retrieve – dissolved in water, oxide of arsenic. Often to obtain applied the simultaneous oxidation and hydrolysis of trialkylaluminium heated to 50° C with a solution of hydrogen peroxide. At the same time from the reaction mixture withdrawn water and alcohol. The solution was then evaporated and have been exposed to arsenic acid of high purity. In the nature of the raw material for the production of arsenic acid are minerals: arsenolite and arsenopyrite, deposits which are rich in Chelyabinsk and Chita region of the Russian Federation.
Application H3AsO4
Given the fact that ortesisacomo acid is one of the strongest poisons. Its application in industry and everyday life is limited. More common salt-arsenate, whose toxicity is much less than thatmost H3AsO4. In the woodworking industry along with zinc sulphate and the sodium salt of pentachlorophenol, arsenic acid used for the treatment of wood. This method minimizes losses from destruction of cellulose fungal infections and beetles larvae borers. In medicine H3AsO4 Is used in the composition of the drug “APSCO” for the treatment of protozoal infections such as giardiasis, balantidiasis, isosporous.

It Should be noted that the infection rate of the population of these infections in recent years dramatically increased. There are several reasons – for example, infection through eating food containing spores of protozoa, through insect bites or through sexual contact. Arsenic acid is used as starting material in processes for the production of optical glasses, as well as in electrical engineering. The derived H3AsO4 is its sodium salt, is successfully used in dermatology, and Phthisiology. Arsenic compounds have been used in dentistry (ARSENICAL paste) as a drug used to reduce pain sensitivity of an inflamed nerve in his removal from the root canal.
The Action of acid on the human body
As mentioned earlier, H3AsO4 Is included in the second class of danger-highly dangerous substances. The lethal dosage is considered as the most acid and its salts in the range of 15 to 150 mg per kilogram of body weight of a person. Along with General toxic effect of arsenic acid induces necrosis of the skin and mucous membranes of internal organs: lungs, stomach, intestines.
In the laboratory when conducting experiments with arsenate and H3AsO4 Be sure to use protective gloves, and the experiments are performed under the hood. In the case of intoxication at the level of the cell breaks down its enzyme system due to inactivation of enzymes. In humans poisoned with arsenate leads to paresis and even paralysis. In Oncology during chemotherapy failure to comply with the dosing regimen of drugs recorded cases of poisoning merceolog and naverstam. First aid for poisoning by salts of arsenic acid consists in immediate gastric lavage (for example, a solution of unithiol or preparations of silicon dioxide).

In Order to prevent acute renal failure, prescribed hemodialysis. As an antidote, except a 5% solution of unitiol, you can use the antidote Strezhevskoi. Before the arrival of emergency at home to reduce the level of intoxication you can apply citric acid solution, and then induce vomiting and gastric lavage. All treatment measures should be subject to strict bed rest under a doctor's supervision.
Article in other languages:

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Blockhead: the meaning of words and interpretation
When we do something wrong, we usually shout: “What are you doing, you knob!”. The meaning of the word, which we call in case of failure, we know without a dictionary. But still, sometimes you can't bring into evidence...
Central correlation of the jaws: definition, methods
In prosthetic dentistry used the term "occlusion". It is understood interdigitation. There are 4 main occlusion and many intermediate. The former include the Central, anterior and 2 lateral. Central occlusion is characterized by t...
For many, OGE – the first serious test, but why is it necessary? The basic state exam required for evaluation of each student's performance over the past nine years of study, it is clear to all. Good results for this exam &n...
How to arrange a bibliography?
the List of references is an integral part of any intellectual work, whether it be a school report or professorial dissertation. To its design, certain rules apply that must be observed in order to avoid difficulties with the supe...
Mass communication as leverage
In the late 20th century the concept of mass communication is widespread. There is a very clear connection between technical possibilities, quality and ways of communication. However, this social phenomenon has deep roots.Even in ...
Children Of Alexander 1. Alexander Pavlovich 1: the reign, personal life, biography
the Emperor Alexander I was the grandson of Catherine the great from her only son Paul Petrovich and the German Princess Sophia of württemberg, in Orthodoxy Maria Feodorovna. He was born in St. Petersburg on 25 Dec 1777. Named in ...





















Comments (0)
This article has no comment, be the first!