Now - 12:40:04
Carbon is... carbon Atom. Weight of carbon
One of the most surprising elements that are able to form a huge variety of compounds of organic and inorganic nature, is carbon. It is so unusual properties of the element that Mendeleev even predicted him a great future, talking about not yet disclosed the specifics.
Later it was confirmed practically. It became known that he is the main nutrient element of our planet, included in the composition of all living beings. In addition, can exist in forms that are radically different in all respects, but consists only of carbon atoms.
In General, the characteristics of this structure are many, and we will try to understand the article.
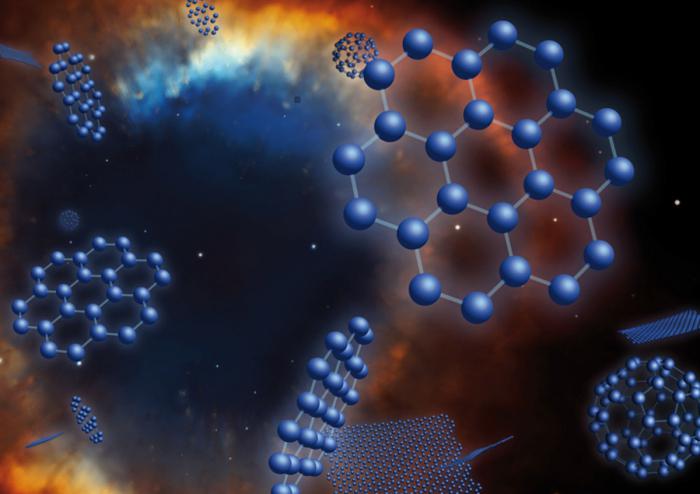
Carbon: the formula and the position in table of the elements
In the periodic table, the element carbon is in the IV (new pattern 14) group main sub-group. Its number 6, and atomic weight 12,011. The callout for know tells me its Latin name - carboneum. There are several different forms in which carbon exists. The formula therefore it is different and depends on the specific modification.
But for writing equations of the reactions, the specific designation, of course. In General, when we refer to the substance in its pure form, adopted the molecular formula of carbon, without indexing.
History of discovery
This element is known since the most ancient times. After all, one of the most important minerals in nature, it is coal. Therefore, for the ancient Greeks, Romans and other peoples secret, he was not.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Apart from this variety, also used diamonds and graphite. With latest a long time there were many complicated situations, often without analysis of the composition for graphite took such compounds like:
- Silver-lead;
- The iron carbide;
- A sulphide of molybdenum.
They were All painted black and therefore considered graphite. Later, this misunderstanding was clarified, and this form of carbon was itself.
Since 1725 the great commercial importance of diamonds, and in 1970 developed the technology of obtaining them by artificial means. In 1779, thanks to the work of Carl Scheele, studied the chemical properties that shows carbon. This was the beginning of a number of important discoveries in the field of this element was the basis for clarification of all its unique features.

The Isotopes of carbon and distribution in nature
Despite the fact that the item in question is one of the most important nutrient, its total content in the mass of the earth's crust is 0.15 %. So comes from the fact that it is under constant circulation, the natural cycle in nature.
In General, you can name a few compounds mineral character, containing carbon. These are natural rocks, such as:
- The Dolomites and limestones;
- Anthracite;
- Shale oil;
- Natural gas;
- Coal;
- Oil;
- Brown coal
- Peat
- Bitumen.
In Addition, we should not forget about living beings, which are just a store of carbon. After all, they formed proteins, fats, carbohydrates, nucleic acids, and therefore the most vital structural molecules. In General, the conversion of dry body weight of 70 kg, 15 are located in the pure element. And so every person, not to mention animals, plants and other beings.
If we consider the composition of air and water, i.e. the hydrosphere as a whole and the atmosphere, here there is a mixture of carbon-oxygen, expressed by the formula CO2. Dioxide or carbon dioxide - one of the main gases that make up air. In this form the mass fraction of carbon is 0,046%. More dissolved carbon dioxide in ocean waters.
The Atomic weight of carbon as an element is 12,011. It is known that this value is calculated as the arithmetic mean between the atomic weights of all the natural isotopic species, given their prevalence (in percentage). It is the same with the substances in question. There are three main isotope, which is carbon. This:
- 12With its mass fraction in the vast majority is 98,93 %;
- 13Of 1.07 %;
- 14 - radioactive, the half-life of 5700 years, resistant betta-emitter.
In the practice of determining the geochronological age of the samples is widely used radioactive isotope 14, which is an indicator, due to its long period of decay.

Allotropic modifications of the element
Carbon is an element, which is in the form of simple substance exists in several forms. That is, it is able to form the largest of the known number of allotropic modifications.
1. Crystalline variations exist in the form of solid structures with the correct arrays of atomic types. This group includes such varieties, kak:
- Almaty;
- Fullerene;
- Gravity;
- Carbine;
- Lonsdeylite;
- A carbon fiber tube.
They All differ in the structure of a crystal lattice, whose nodes is a carbon atom. Hence completely unique, not similar properties, both physical and chemical.
2. Amorphous forms - their forms to a carbon atom that is part of thesome natural compounds. That is not pure varieties, but with admixtures of other elements in small quantities. This group includes:
- Activated carbon;
- Stone and wood;
- Soot;
- Carbon nanopen;
- Anthracite;
- Glassy carbon;
- Technical kind of stuff.
Also combined features of the structure of the crystal lattice, explaining and proving properties.
3. Carbon compounds in the form of clusters. Such a structure in which the atoms are connected into a special hollow inside conformation, filled with water or with nuclei of other elements. Examples:
- Carbon nano;
- Astralene;
- Diapered.

Physical properties of amorphous carbon
Due to the large variety of allotropic modifications, to find some physical properties for carbon is difficult. It's easier to talk about a specific form. So, for example, amorphous carbon has the following characteristics.
- The basis of all forms - crystalline varieties of graphite.
- High capacity.
- Good conductive properties.
- The Density of carbon is about 2 g/cm3.
- When heated above 1600 0There is a transition in graphite form.
Soot, charcoal and stone varieties are widely used for technical purposes. They are not the manifestation of modification of carbon in its pure form, but contain it in very great numbers.
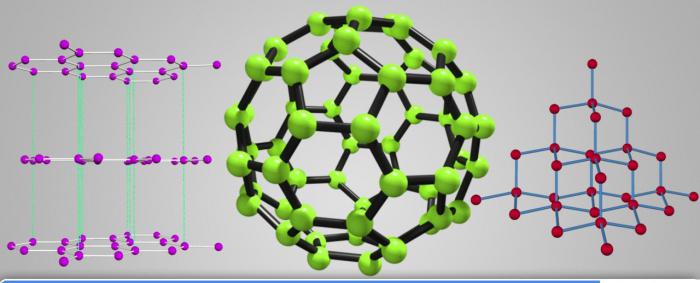
Crystalline carbon
There are several options in which a carbon substance that forms the right crystals, where the atoms are connected in series. The result is the formation of the following modifications.
- Diamond. The structure is cubic, which connects four of the tetrahedron. As a result, all covalent chemical bonds per atom maximally dense and durable. It explains physical properties: carbon density 3300 kg/m3. High hardness, low heat, no electric conductance is the result of the structure of the crystal lattice. There are technically received the diamonds. Formed in the transition graphite in the following modification under the influence of high temperature and certain pressure. In General, the melting point of diamond is as high as the strength is about 3500 0C.
- Graphite. The atoms are arranged like the structure of the previous substance, but there is a saturation of only three ties, and the fourth becomes longer and less sturdy, it connects layers of hexagonal rings of the lattice. The result is that graphite is soft, greasy to the touch the substance of black color. It has good electrical conductivity and has high melting point - 3525 0C. Capable of sublimation - the sublimation from solid to gas, bypassing the liquid (at the temperature 3700 0). Carbon density - of 2.26 g/cm3, Which is far below that of diamond. It explains their different properties. Due to the layered structure of the crystal lattice, it is possible to use graphite for manufacture of slate pencils. When holding the paper flakes and the peel is left on the paper trail in black.
- Fullerene. Open was only in the 80-ies of the last century. Represent modifications in which the carbons are connected in a special closed convex structure having a Central void. The shape of the crystal is a polyhedron, the proper organization. The number of atoms is even. The most known form of fullerene C60. Samples of this substance were found during research:
- Meteorites;
- Bottom deposits;
- Folgorito;
- Shungites;
- Outer space, which contained gases.
All the varieties of crystalline carbon are of great practical importance, as have a number of useful properties in the technique.
Chemical activity
Molecular carbon exhibits low chemical activity due to their stable configurations. To get him to react is possible only by giving the atom additional energy and forcing the electrons in the outer level to steam. At this point, the valence is equal to 4. Therefore in compounds it has an oxidation state + 2, + 4, - 4.
Almost all reactions with simple substances like metals and non-metals, occur under the influence of high temperatures. The item in question can be both an oxidizing agent and a reducing agent. However, the latter properties are expressed it is particularly strong, it is the basis for its use in metallurgical and other industries.
In General, the ability to enter into chemical interaction depends on three factors:
- Dispersion of carbon;
- Allotropic modifications;
- Temperature reactions.
Thus, in some cases, the interaction with the following substances:
- Nonmetals (hydrogen, oxygen);
- Metals (aluminum, iron, calcium and other);
- Metal oxides and their salts.
With acids and alkalis do not react with Halogens is very rare. The most important properties of carbon the ability to form long chain between them. They may withdraw cycle, forming branching. So there is a formation of organic compounds, which today number in the millions. The basis of thesecompounds of two elements - carbon, hydrogen. Also, the composition can contain additional atoms of oxygen, nitrogen, sulfur, Halogens, phosphorus, metals, etc.
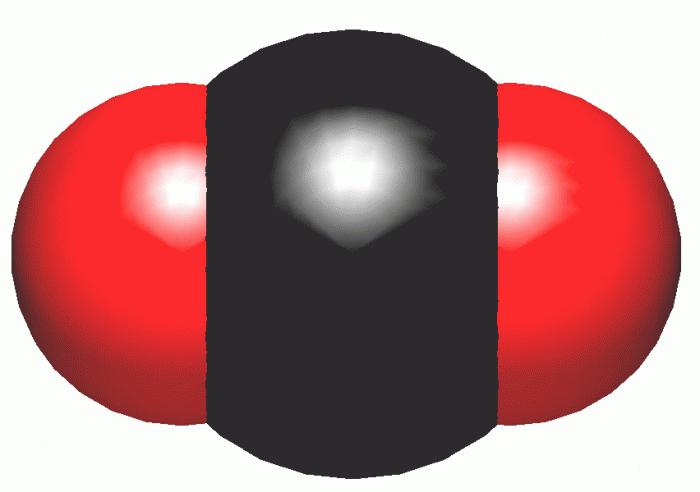
Main links and their characteristics
There are many different compounds containing carbon. The most famous of them - the CO2 - carbon dioxide. However, in addition to this oxide, there is also CO - monoxide or carbon monoxide and methoxide3O2.
Among the salts containing this element, the most common are the carbonates of calcium and magnesium. Thus, the calcium carbonate has several synonyms in the title, as found in nature in the form:
- Chalk;
- Marble
- Limestone;
- Dolomite.
The importance of carbonates of alkaline earth metals is that they are active participants in the processes of formation of stalactites and stalagmites and groundwater.
Carbonic acid is another compound that forms carbon. Its formula is N2CO3. However, in conventional form, it is extremely unstable and immediately breaks up in solution into carbon dioxide and water. Therefore known only in its salts, but it is not the solution.
The Halides of carbon are obtained mostly by indirect means, since direct synthesis only at very high temperatures and low product yield. One of the most common - CCL4 carbon tetrachloride. Toxic compound, when inhaled, are capable to cause a poisoning. Get in the photochemical reactions of the radical substitution of the hydrogen atoms in methane.
The metal Carbides - compounds of carbon in which it exhibits the oxidation state 4. It is also possible the existence of associations with boron and silicon. The main feature of the carbides of some metals (aluminum, tungsten, titanium, niobium, tantalum, hafnium) is a high strength and excellent electrical conductivity. Boron carbide4Is one of the hardest substances after diamond (9,5 Mohs). These compounds are used in engineering and chemical industry, as the sources of hydrocarbons (calcium carbide with water leads to the formation of acetylene and calcium hydroxide).
Many metal alloys are made using carbon, greatly improving their quality and technical specifications (steel - alloy of iron with carbon).
Individual attention, numerous organic compounds of carbon in which it is a fundamental element, able to connect with the same atoms in long chains with different structures. These include:
- Alkanes;
- Alkenes;
- Arena;
- Whites;
- Carbohydrates;
- Nucleic acids
- Alcohol
- Carboxylic acids many other substance classes.
Carbon
The Value of carbon and its allotropic modifications in a person's life is very great. You can call some of the most global sectors, to make it clear that this is true.
- This item forms all types of organic fuel, from which the person receives the energy.
- Iron and steel industry uses carbon as a strong reductant for producing metals from their compounds. Here find wide application carbonates.
- Construction and chemical industry consume vast amounts of carbon compounds for the synthesis of new substances and obtaining of the necessary ingredients.

You can Also call such industries as:
- The nuclear industry;
- Jewelry;
- Technical equipment (grease, high-temperature crucibles, pencils etc.);
- Determination of the geological age of the rocks is the radioactive tracer 14C;
- Carbon is a great adsorbent that allows it to be used for the manufacture of filters.
Cycle in nature
The Mass of carbon available in nature, included in constant circulation, which takes place cyclically every second around the globe. Thus, the atmospheric source of carbon - CO2 is absorbed by plants and released all living beings in the breathing process. Once in the atmosphere, it is absorbed again, and so the cycle continues. The withering away of organic residues leads to the release of carbon and the accumulation of it in the ground, where it then is absorbed again by living organisms and is released into the atmosphere as a gas.
Article in other languages:
AR: https://tostpost.com/ar/education/1741-carbon-is-carbon-atom-weight-of-carbon.html
BE: https://tostpost.com/be/adukacyya/3019-vuglyarod---geta-atam-vuglyarodu-masa-vuglyarodu.html
ES: https://tostpost.com/es/la-educaci-n/3022-el-carbono-es-el-tomo-de-carbono-masa-de-carbono.html
HI: https://tostpost.com/hi/education/1741-carbon-is-carbon-atom-weight-of-carbon.html
JA: https://tostpost.com/ja/education/1740-carbon-is-carbon-atom-weight-of-carbon.html
KK: https://tostpost.com/kk/b-l-m/3020-k-m-rtek---b-l-atom-k-m-rtek-bolady-massasy-k-m-rteg.html
PL: https://tostpost.com/pl/edukacja/3023-w-giel---to-atom-w-gla-masa-w-gla.html
PT: https://tostpost.com/pt/educa-o/3020-o-carbono-um-tomo-de-carbono-a-massa-de-carbono.html
TR: https://tostpost.com/tr/e-itim/3025-karbon---bu-karbon-atomu-a-rl-k-karbon.html
UK: https://tostpost.com/uk/osv-ta/3022-vuglec---ce-atom-vuglecyu-masa-vuglecyu.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Ekaterinburg, honey. College: description, specialties, features of receipt and feedback
a Newborn baby, born, first sees the medical professional. This specialist accompanies a person throughout his life, helps to cope with illnesses, taking care of his health. People say that doctors are born, not made. And indeed i...
The rate of reaction in chemistry: definition and its dependence on various factors
the reaction Rate is a value which shows the concentration of the reactants over a period of time. In order to estimate its size, you must change the initial conditions of the process. Homogeneous interaction the rate of the react...
What is the difference of myth from tales and legends?
Unlike myth from fairy tales obvious. For the modern person, both types of narrative talk about the wonders, the adventures of the characters (people, animals or gods), endowed with supernatural qualities. However, if you look clo...
What studies Ethnography? The task of Ethnography
this article outlines the answer to the question about what studying Ethnography. We will tell about the science, we point out some of its features, explain its relevance and significance.where to start the answer to the question ...
State universities of St. Petersburg. A rating of universities of St. Petersburg
After graduation, graduates have no time to relax, because in front of them waiting for a new Chapter in the life of the students. But where better to do that? For example, the state universities of St. Petersburg is ready to acce...
Characteristics of the image of Dorian gray (Oscar Wilde, "the picture of Dorian gray")
a novel by Oscar Wilde, as the writer's life, causing a lot of debate and conflicting opinions. What only epithets was awarded the work, where “immoral” and “ruin” is still relatively modest.that is why the...






















Comments (0)
This article has no comment, be the first!