The rate of reaction in chemistry: definition and its dependence on various factors
The reaction Rate is a value which shows the concentration of the reactants over a period of time. In order to estimate its size, you must change the initial conditions of the process.
Homogeneous interaction
The rate of the reaction between some compounds present in one aggregate form, depends on what is the volume of the taken substances. From a mathematical point of view it is possible to Express the dependence between the velocity of a homogeneous process and the change in concentration per unit time.
An Example of such interaction can be considered as the oxidation of nitric oxide (2) oxide of nitrogen (4).
Heterogeneous processes
The Rate of reaction for the source of substances in different aggregate States, is characterized by the number of moles of initial reactants per unit area per unit time.
Heterogeneous interactions characteristic of systems that have a different physical state.
Summing up, we note that the rate of reaction shows the change in the number of moles of the initial reagents (products of interaction) over a period of time, per unit surface section of phases or per unit volume.
Concentration
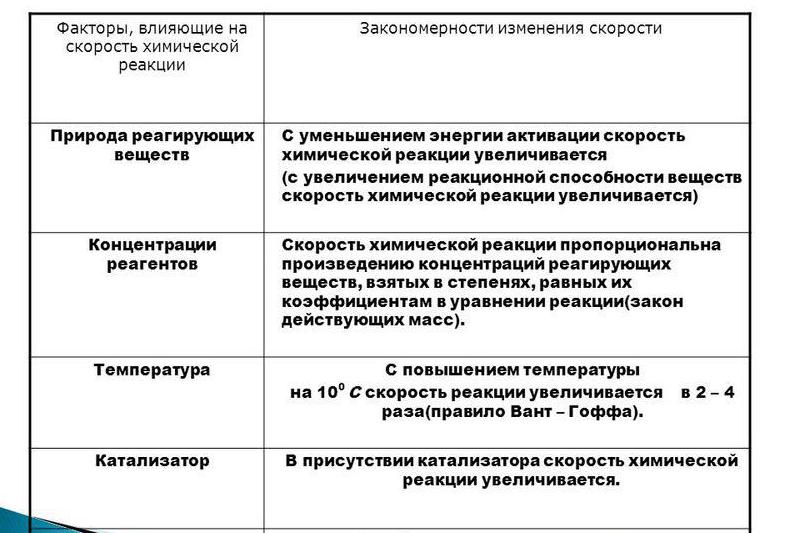
Consider the main factors affecting the reaction rate. Let's start with concentration. This dependence is expressed by the law of mass action. Between the product of concentrations of substances interacting, taken in the extent of their stereochemical factors, and speed of reaction, there is a directly proportional relationship.
Consider the equation AA +BB = CC + DD where A, b, C, D – are liquids or gases. For a given process, the kinetic equation can be written taking into account the coefficient of proportionality, which for each interaction has its own value.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
As the primary reasons for the growth speed can be noted the increase in the number of collisions of the reacting particles per unit volume.
Temperature
Consider the effect of temperature on the reaction rate. The processes that occur in a homogenous system, is possible only if the particle impact. But not all collisions lead to the formation of the reaction products. Only in the case when the particles are increased energy. When heating of the reactants, an increase of the kinetic energy of the particles, increasing the number of active molecules, so the observed increase in reaction rate. The connection between the temperature indicator and the speed of the process is determined by the rule of van't Hoff: each increase in temperature of 10°C leads to an increase of the speed of the process 2-4 times.
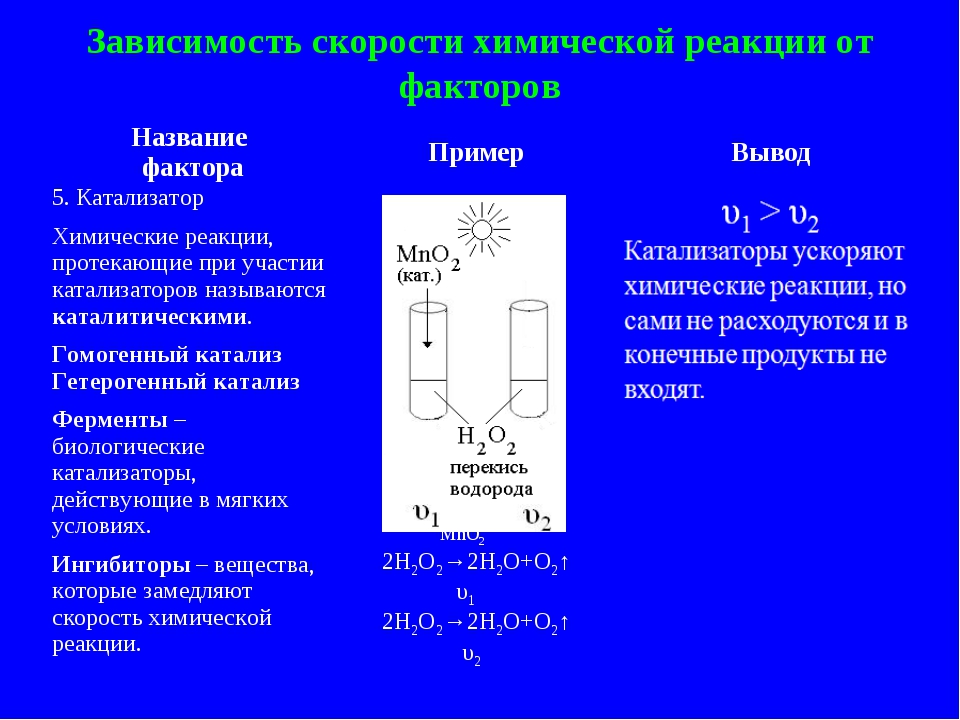
The Catalyst
Considering the factors affecting the speed of reaction, focus on the substances that can increase the speed of the process, that is, on the catalysts. Depending on the state of aggregation of the catalyst and the reacting substances, there are several types of catalysis:
- Homogeneous appearance, in which the reagents and catalyst have the same physical state;
- Heterogeneous when the reactants and catalyst are in same phase.
As examples of substances that accelerate the interaction, we can distinguish Nickel, platinum, rhodium, palladium.
Inhibitors of the considered substances that slow down the reaction.

Contact Area
What else depends on the rate of reaction? Chemistry is divided into several sections, each of which dealt with certain processes and phenomena. In the course of physical chemistry examines the relationship between contact area and speed of the process.
In order to increase the area of contact of the reagents, they are grinded to a certain size. Fastest of all is the interaction in solution, which is why many reactions are carried out in an aqueous medium.
The grinding of solids should comply with the measure. For example, in the conversion of pyrite (iron sulfite) to dust in the kiln takes place sintering of the particles, which adversely affects the speed of the oxidation process of this compound, decreases the yield of sulfur dioxide.
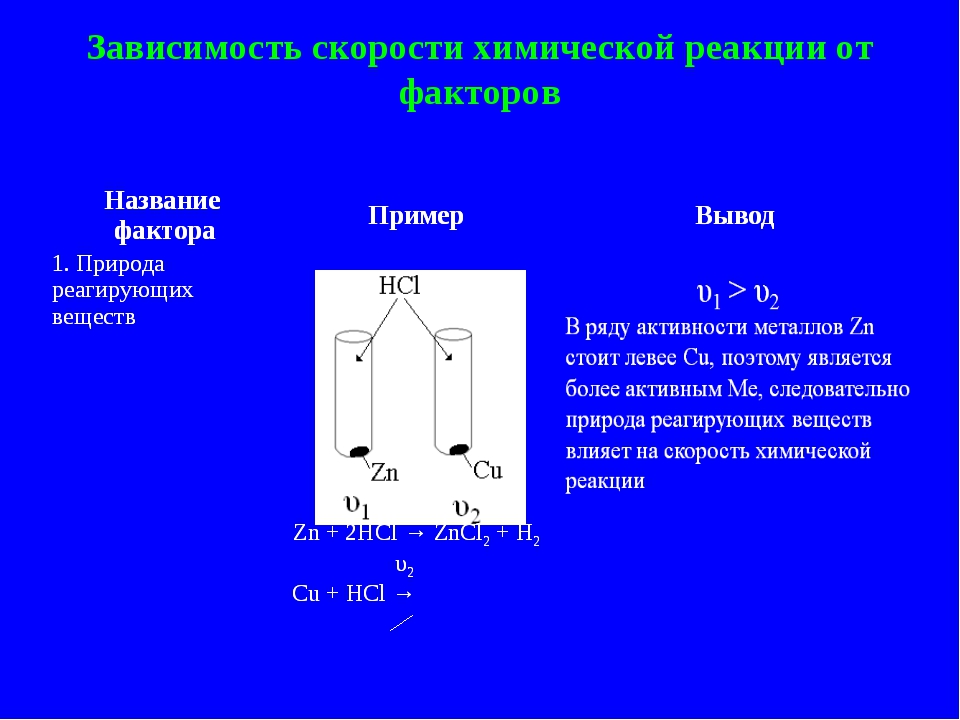
Reagents
Let's Try to understand how to determine the rate of reaction depending on the ingredients interact? For example, the active metal located in the electrochemical series Beketov to hydrogen capable of reacting with the acid solutions, and those that are after N2, have no such ability. The reason for this phenomenon lies in the different chemical activity of metals.

The Pressure
As with this amount linked to the rate of reaction? Chemistry is the science that is closely related to physics, so the dependence is directly proportional, it is governed by the gas laws. There is a direct relationship between the variables. And to understand what the law determines the rate of chemical reactions, it is necessary to know the aggregate state andthe concentration of the reactants.
Kinds of speeds in chemistry
Decided to allocate instantaneous and average. The average speed of the chemical interaction is determined as the difference between the concentrations of the reacting substances during the time period.
The resulting value has a negative value in the case when there is a decrease in the concentration of positive – with the increase in the concentration of products of interaction.
The True (instantaneous) value is such an attitude in a certain time unit.
In SI units the speed of a chemical process is expressed in [mol × m-3×-1].
Tasks in chemistry
Let us Consider some examples of tasks associated with the definition of velocity.
Example 1. In a vessel mix the chlorine and hydrogen, then the mixture is heated. After 5 seconds the concentration of hydrogen chloride became 0.05 mol/DM3. Calculate the average rate of formation of hydrogen chloride (mol/DM3).
You Must define the change in the concentration of hydrogen chloride after 5 seconds after the interaction, by subtracting from the final concentration of the starting value:
C(HCl) = c2 - c1 = 0,05 - 0 = 0.05 mol/DM3.
Compute the average rate of formation of hydrogen chloride:
V = 0,05/5 = 0,010 mol/DM3 ×C.
Example 2. In the vessel, which amounts to 3 DM3, the following process occurs:
C2H2 + 2H2=C2H6.
The Initial mass of hydrogen-1 g. After two seconds after the beginning of interaction the mass of the hydrogen has acquired a value of 0.4, Calculate the average speed for ethane (mol/DM3×s).
The Mass of hydrogen that reacts, is determined as the difference between the initial value and the end number. It is 1 - 0,4 = 0,6 (g). To determine the number of moles of hydrogen, it is necessary to divide it by the molar mass of the gas: n = a 0.6/2 = 0.3 mol. According to the equation 2 mole of hydrogen to produce 1 mole of ethane, therefore, of 0.3 mol N2 obtained from 0.15 mol of ethane.
Determine the concentration of the formed hydrocarbons obtained 0.05 mol/DM3. Then you can calculate the average velocity of its formation: =0,025 mol/DM3 ×C.

Conclusion
On the rate of chemical interaction is influenced by various factors: the nature of the reactants (the activation energy), their concentration, the presence of a catalyst, the degree of crushing, pressure, type of radiation.
In the second half of the nineteenth century by Professor N. N. Beketov had made the assumption that between the mass of the initial reagents and the duration of the process is communication. This hypothesis was confirmed in the law of mass action, established in 1867, the Norwegian chemists: P. Vahe and K. Goldberg.
Studies on the mechanism and speed of flow of the different processes involved in physical chemistry. The simplest processes in one stage, referred to as monomolecular processes. Complex interactions involve several successive elementary interactions, so each stage is considered separately.
In order with minimal energy costs you can count on getting the maximum yield of the reaction products, it is important to consider the basic factors which influence the process flow.
For Example, to speed up the process of decomposition of water in simple substances required in the catalyst, which performs the role of manganese oxide (4).
All the nuances associated with the selection of reagents, selection of the optimum pressure and temperature, concentration of reactants, considered in chemical kinetics.
Article in other languages:

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
What is the difference of myth from tales and legends?
Unlike myth from fairy tales obvious. For the modern person, both types of narrative talk about the wonders, the adventures of the characters (people, animals or gods), endowed with supernatural qualities. However, if you look clo...
What studies Ethnography? The task of Ethnography
this article outlines the answer to the question about what studying Ethnography. We will tell about the science, we point out some of its features, explain its relevance and significance.where to start the answer to the question ...
State universities of St. Petersburg. A rating of universities of St. Petersburg
After graduation, graduates have no time to relax, because in front of them waiting for a new Chapter in the life of the students. But where better to do that? For example, the state universities of St. Petersburg is ready to acce...
Characteristics of the image of Dorian gray (Oscar Wilde, "the picture of Dorian gray")
a novel by Oscar Wilde, as the writer's life, causing a lot of debate and conflicting opinions. What only epithets was awarded the work, where “immoral” and “ruin” is still relatively modest.that is why the...
The meaning of the word Pharaoh in ancient Egyptians meant much more than the ruler
the meaning of the word Pharaoh in the majority of Russian dictionaries is explained in this way – this is the title of ancient Egyptian rulers or persons with Royal features.Origin of termthe Ancient Egyptians were unwillin...
Than the Gregorian calendar differs from the Julian calendar. Julian calendar in Russia
For all of us the calendar is a thing familiar and even ordinary. It is the oldest human invention records the days, numbers, months, seasons, periodicity of natural phenomena, which is based on a system of movement of heavenly bo...





















Comments (0)
This article has no comment, be the first!