Now - 05:30:25
Atom in chemistry is... model of the atom. The structure of the atom
Thoughts about the nature of the surroundings began to visit mankind long before the time of flowering of modern civilization. First, people have speculated about the existence of some higher powers which, as they believed, predetermined all existence. But pretty soon the philosophers and the priests began to ponder what, in fact, is the very fabric of this existence. Theories were many, but in a historical perspective has become the dominant atomic.
 What is an atom in chemistry? This and all other related topics we will discuss in this article. I hope that in it you'll find the answers to all your questions.
What is an atom in chemistry? This and all other related topics we will discuss in this article. I hope that in it you'll find the answers to all your questions.
The founder of the atomic theory
How to start the first lesson of chemistry? The structure of the atom – this is the main issue. You probably remember that the word "atom" is translated from Greek as "indivisible". Now, many historians believe that the first advanced the theory, which read about some of the tiny particles that comprise all things, Democritus. He lived in the fifth century BC.
Unfortunately, this is an outstanding thinker, virtually nothing is known. Did not reach us no written source at the time. But because the ideas of the greatest scientist of his time, we have to learn solely from the writings of Aristotle, Plato and other Greek thinkers.
So, our topic is "the atom". In chemistry not all had the highest scores, but many remember that all the conclusions of the ancient scholars was built solely on inferences. Democritus was not the exception.
As argued by Democritus?
His Logic was simple, yet brilliant. Imagine that you have the sharpest knife in the world. You take an Apple, for example, and then begin to cut two halves into quarters, divide them again... in short, sooner or later you will get chunks such meager thickness that continue to divide them already will be. So this is the indivisible atom. In chemistry, this statement was considered true almost to the end of the 19th century.
From Democritus to modern concepts
It Should be noted that from the ancient Greek concepts of the microcosm remained only one word "atom". Now every schoolboy knows that the world around us consists of much more fundamental and small particles. In addition, from the point of view of modern science, the theory of Democritus was not more than a purely hypothetical calculation, not supported by absolutely no evidence. However, in those days there were no electron microscopes, so to prove his innocence in other ways, the thinker would not have worked.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
The First suspicion that Democritus was right, appeared to chemists. They quickly discovered that many of the substances in the reactions disintegrate into smaller components. In addition, chemists have deduced a strict regularities of these processes. So, they noticed that for obtaining the water requires eight mass fraction of oxygen and one of hydrogen (Avogadro's law).
In the Middle ages any materialistic doctrine, including the theory of Democritus, dissemination and development of the to could not in principle. And only in the XVIII century, scientists once again returning to the atomic theory. By the time the chemist A. Lavoisier, our great Lomonosov and talented English physicist J. Dalton (about which we will talk separately) has already been convincingly demonstrated to their colleagues the reality of the existence of atoms. It should be emphasized that even in the enlightened 18th century for a long time, the atomic theory many outstanding minds of the time never seriously considered.
Whatever it was, but even these great scientists had not yet advanced theories of the structure of the atom, as it was considered a single and indivisible particle, the basis of everything.
Unfortunately, chemical tests could not clearly prove the reality of conversion of atoms of some substances into others. But the fundamental science in studying the structure of atoms was precisely chemistry. Atoms and molecules has long been studied one of the ingenious Russian scientist, without which it is impossible to imagine modern science.
The Teachings of D. I. Mendeleev
 A Huge role in the development of the atomic doctrine was played by D. I. Mendeleev, who in 1869 established its brilliant periodic system. For the first time the scientific community was the theory, which not only rejected, but also reasonably complement all the assumptions of the materialists. Already in the 19th century, scientists were able to prove the existence of electrons. All of these conclusions forced the best minds of the 20th century a serious study of the atom. In chemistry this time was also marked by many discoveries.
A Huge role in the development of the atomic doctrine was played by D. I. Mendeleev, who in 1869 established its brilliant periodic system. For the first time the scientific community was the theory, which not only rejected, but also reasonably complement all the assumptions of the materialists. Already in the 19th century, scientists were able to prove the existence of electrons. All of these conclusions forced the best minds of the 20th century a serious study of the atom. In chemistry this time was also marked by many discoveries.
But the doctrine Mendeleev valuable not only to it. Still, it remains unclear how it was formed, the atoms of different chemical elements. But the great Russian scientist was able to convincingly prove that all of them without exception are in close relationship with each other.
Opening of Dalton
But being able to interpret multiple disparate data could only John Dalton, whose name is forever etched in the open to them by law. Usually, the researchers examined only the behavior of gases, but the range of his interests was much wider. In 1808 he began the publication of his new fundamental work.
Dalton suggested that each chemical element corresponds to a certain atom. But the scientist,as Democritus many centuries before him, still believed that they are completely indivisible. His drafts are a lot of schematic drawings, in which the atoms are represented as simple balls. This idea originated more than 2,500 years ago, lasted almost to our time! However, only relatively recently was opened a really deep structure of the atom. Chemistry (grade 9 in particular) even today is largely guided by the ideas which were first voiced in the 18th century.
Experimental verification of divisibility of atoms
However, until the late 19th century almost all scientists believed that the atom - the limit beyond which there is nothing. They thought that the basis of all creation is exactly what it is. This was facilitated by various experiments anyway, but changed only the molecules, while with the very atoms of the substances does not happen nothing, which would not have been able to explain simple chemistry. The structure of the carbon atom, for example, remains absolutely the same even in different allotropic States.
In short, a long time there was absolutely no experimental data that at least indirectly confirmed the suspicion of some scholars that there are some more fundamental particles. Only in the 19th century (not least thanks to the experiments by the curies), it was proved that under certain conditions the atoms of some elements can become more. These discoveries formed the basis of modern ideas about the world around us.
Raisins and puddings
In 1897, George. Thomson, English physicist, it was found that every atom has a certain number of negatively charged particles, which he called "electrons". In 1904, the scientist created the first atomic model, which is better known under the designation of "plum pudding". The name very accurately reflects the essence. According to the theory of Thomson, the atom in chemistry – a “vessel” with evenly distributed in it charge, and electrons.
Note that a similar model had been in circulation even in the 20th century. It later turned out that she was completely wrong. But it was the first conscious attempt of a human (and scientific based) to recreate the surrounding microcosm, proposing a model of the atom, is quite simple and clear.
Experiments Curie
It is considered that the couple Pierre and Marie Curie marked the beginning of atomic physics. Of course, the contribution of these brilliant people, actually sacrifice their health and life should not be underestimated, but their experiences were much more fundamental. Almost simultaneously with Rutherford, they proved that the atom – much more complex and heterogeneous structure. The phenomenon of radioactivity, which they investigated, it was talking about.
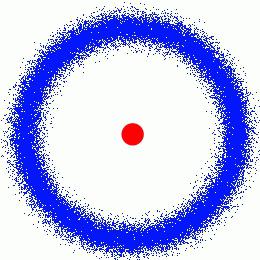 In early 1898, Mary published the first article on radiation. Soon Maria and Pierre Curie proved that in the mixture of chlorides of uranium and radium start to appear other substances, the existence of which doubt the official chemistry. The structure of the atom since then, began to explore in earnest.
In early 1898, Mary published the first article on radiation. Soon Maria and Pierre Curie proved that in the mixture of chlorides of uranium and radium start to appear other substances, the existence of which doubt the official chemistry. The structure of the atom since then, began to explore in earnest.
"Planetary" approach
Finally, Rutherford decided to carry out the bombardment of the atoms of heavy metals α-particles (fully ionized helium). The scientist immediately suggested that light the electrons will not be able to change the trajectory of the particles. Accordingly, the dispersion can be any heavier elements that may be contained in the nucleus of an atom. Note that initially, Rutherford did not claim to change the theory "pudding". This model of the atom was considered to be immaculate.
And because the result, in which almost all particles without problems passed through a thin layer of silver, it is not surprising. That's just it soon became clear that some of the helium atoms deviate by as much as 30°. It was not what was said at the time chemistry. The composition of the atom Thomson assumed uniform distribution of electrons. But this is clearly contrary to the observed phenomenon.
Extremely rare, but still some particles flew at an angle, even 180°. Rutherford was in the deepest perplexity. After all, it is sharply contradicted "pudding", a charge which was supposed to be (according to the theory of Thomson) are distributed uniformly. Therefore, unevenly charged areas, which could repel the ionized helium, was absent.
What are the conclusions of Rutherford?
These circumstances have prompted the scientist to the idea that the atom is almost empty and only concentrated in the centre some education with a positive charge, the nucleus. And there was the planetary model of the atom, the postulates of which is as follows:
- As we already said, in the Central part is the core, and its volume (relative to the size of the atom) is negligible.
- Almost the entire atomic mass and all positive charge are found in the nucleus.
- Around it revolve electrons. It is important that their number is equal to the value of the positive charge.
Paradoxes of theory
 It would be good, but this model of the atom does not explain their incredible resilience. It should be remembered that the electrons move in their orbits with great acceleration. According to the laws of electrodynamics, such a facility should lose its charge. If you take into account the postulates of Newton and Maxwell, electrons generally have to roll to the core, like hail to the ground.
It would be good, but this model of the atom does not explain their incredible resilience. It should be remembered that the electrons move in their orbits with great acceleration. According to the laws of electrodynamics, such a facility should lose its charge. If you take into account the postulates of Newton and Maxwell, electrons generally have to roll to the core, like hail to the ground.
Of Course, nothing in reality is not happening. Any atom not only completely sustainable, but can exist completely unlimited time, and no radiation it will not go. This discrepancy is explained by the fact that the microcosm we try to apply the laws which are valid only in relation to classical mechanics. They, as proved, by the phenomena of atomic scale is absolutely not applicable. But because the structure of the atom (chemistry, grade 11) the authors of the textbooks try to explain as simple as possible words. the
The Doctrine of Boron
The Danish physicist Niels Bohr proved that the microcosm is impossible to extend the same laws, regulations which are fair for macroscopic objects. It was his idea that the microcosm “guided” only quantum laws. Of course, then there was no quantum theory, but Bohr actually was her ancestor, expressing his thoughts in the form of three postulates that “saved” the atom would inevitably lost, if he "lived” according to the theory of Rutherford. It is this theory of the Dane formed the basis of all quantum mechanics.
The Postulates of Bohr
- The First of them reads as follows: any atomic system can only exist in a particular atomic state, and for each of them characterized by a certain energy value (E). If the stationary state of the atom (calm), reject it not.
- The Second postulate says that light radiation of energy occurs only in the case of transition from the state with higher energy is more moderate. Accordingly, the energy released is equal to the difference of values between two stationary States.
Model of the atom Niels Bohr
This semiclassical theory, scientists have proposed in 1913. It is noteworthy that in its basis it is put planetary model of Rutherford, who had described the atom of the substance. We have already said that classical mechanics was contrary to the calculations of Rutherford: according to her, it was assumed that with time the electron must fall to the surface of the atom.
To “around” this is a contradiction, a scientist has introduced a special assumption. Its essence lies in the fact that radiating energy (which would lead to their downfall) the electron can only moving in certain orbits. When driving them on other alleged chemical trajectories of the atoms remained in a passive state. According to the theory, these orbits were those, quantitative motion, which was equal to Planck's constant.
Quantum theory of atomic structure
 As we have said, to date, the course quantum theory of atomic structure. Chemistry in recent years guided solely by it. It is based on four fundamental axioms.
As we have said, to date, the course quantum theory of atomic structure. Chemistry in recent years guided solely by it. It is based on four fundamental axioms.
1. First, the duality (wave-particle nature) of the electron. Simply put, behaves this particle as a material object (a corpuscle), and as a wave. As a particle it has a certain charge and mass. The ability of the diffraction of electrons in common with classical waves. The length of the wave itself (λ) and velocity of particle (v) can be connected to each other by a special ratio of de Broglie wavelength: λ = h / mv. As you might guess, m-the mass of the electron.
2. The coordinate and the velocity of the particle to measure with absolute precision quite impossible. The more precisely defined coordinate, the higher the uncertainty in the speed. As, however, and Vice versa. This phenomenon is called the uncertainty principle, which can be expressed as the following ratio: ∆x∙m∙∆v > )/2. Delta X (∆x) expresses the uncertainty of the position coordinates in space. Accordingly, the Delta V (∆v) displays the speed error.
3. Contrary to all previously prevalent opinions, the electrons do not pass on strictly defined orbits like trains on rails. Quantum theory States that electron can be in any point of space, but the probability is different for each segment.
That part of the space directly around the atomic nucleus in which the probability is maximum is called orbital. Modern chemistry the structure of electronic shells of atoms studying from this point of view. Of course, the schools teach the proper distribution of the electrons in levels, but apparently in reality they differ very different.
4. The nucleus consists of nucleons (protons and neutrons). The ordinal number of the element in the periodic table indicates the number of protons in its nucleus, and the sum of protons and neutrons equal atomic mass. Here's how to explain the structure of atom chemistry of modernity.
The Founders of quantum mechanics
 Note the scientists who made the greatest contribution to the development of such an important industry: the French physicist L. de Broglie, Heisenberg German, Austrian, E. Schrodinger, P. Dirac Englishman. All of these people were subsequently awarded the Nobel prize.
Note the scientists who made the greatest contribution to the development of such an important industry: the French physicist L. de Broglie, Heisenberg German, Austrian, E. Schrodinger, P. Dirac Englishman. All of these people were subsequently awarded the Nobel prize.
How far this plan went chemistry? The structure of the atom most of the chemists of those years believed, quite simple: a only to 1947, finally recognized the reality of the existence of elementary particles.
Some insights
Generally, when I create a quantum theory without mathematics, since these processes can only be calculated usingthe most complicated calculations. But the main difficulty is not the point. The processes that are described by this theory, not only inaccessible to our senses, despite all modern scientific technique, but also imagination.
No one, even approximately cannot imagine the processes in the microcosm as they are quite similar to all the phenomena that we observe in the macrocosm. Just think: recent discoveries give reason to assume that quarks, neutrinos and other fundamental particles exist in nine-dimensional (!) dimension. As someone who lives in three-dimensional space, can even begin to describe their behavior?
At the moment, we can only rely on math and the power of modern computers that may be used for modeling of the microworld. Significantly helps and chemistry: structure of the atom will probably be revised after recently, scientists working in this area, reported the discovery of a new type of chemical bond.
Current understanding of the structure of the atom
If you have read all above, you probably will be able to tell what is the current idea about the structure of atoms. But we explain that it is somewhat modified theory of Rutherford, complemented by an invaluable postulates of Niels Bohr. Simply put, today it is believed that the electrons move in a chaotic, blurred trajectories near the nucleus that consists of neutrons and protons. That part of the space around it in which the appearance of an electron most likely is called the orbital.
While it is not possible to say exactly how they will change our understanding of the structure of the atom in the future. Every day scientists are working on a penetration into the secrets of the micro world: the LHC (Large hadron Collider), the Nobel prize in physics – all this is the result of these surveys.
But even now we do not represent or approximate picture of what else are you hiding atoms. It is clear that the atom itself in the scale of the microcosm – a huge apartment house in which we examined is that the first floor, and even then not completely. Almost every year there are reports of the ability to open all new and new elementary particles. When the process of investigation of atoms will be completed, today, will undertake to predict anybody.
 Suffice it to say that our perceptions of them started to change only since 1947, when they were discovered the so-called V-particles. Before that people only slightly deepened the theory on which the 19th century was based chemistry. The structure of the atom – a fascinating mystery, the solving of which occupied the best minds of mankind.
Suffice it to say that our perceptions of them started to change only since 1947, when they were discovered the so-called V-particles. Before that people only slightly deepened the theory on which the 19th century was based chemistry. The structure of the atom – a fascinating mystery, the solving of which occupied the best minds of mankind.
Article in other languages:
BE: https://tostpost.com/be/adukacyya/33083-atam-u-h-m---geta-madel-atama-budova-atama.html
KK: https://tostpost.com/kk/b-l-m/33409-atom-himiya---b-l-model-atom-fizikasy-atom-rylysy.html
PL: https://tostpost.com/pl/edukacja/34457-atom-w-chemii---to-model-atomu-budowa-atomu.html
TR: https://tostpost.com/tr/e-itim/29835-atom-kimya-atom-modeli-atomun-yap-s.html
UK: https://tostpost.com/uk/osv-ta/33603-atom-u-h-m---ce-model-atoma-budova-atoma.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Where can lead continuing education
From birth to death man has constantly to learn in order to survive, to adapt to a changing reality, to find and know yourself and wise to live your life. The concept of lifelong education, learning, self-improvement is present in...
Cruciferous plants and their characteristics
All angiosperms (flowering) plants are divided into monocots and dicots. First class includes such families as Liliaceae, onions, grasses, orchids, palms, Araceae, Cyperaceae. The second includes all the rest, for example, rozotsv...
Introductory turn. Introductory words, phrases and sentences. Setting punctuation
In his speech, people often use introductory design to show their attitude to what they tell you. The introductory turnover must be allocated by commas, and in speech such turnover should be allocated to the intonation. Let us con...
On which river is the Kazan. Natural attractions of Kazan
Kazan-capital of Tatarstan. The city has millennial history, unique culture, developed economy, is a scientific center of the Republic. On site is a large port. On which river is the Kazan-Volga or Kazanka?History of nameFew peopl...
Antonyms – opposed to each other in meaning, but belong to the same part of speech of the word. They have different writing and sound. To determine the value of one of the antonym is very simple through the other, enough to ...
Who was the first inventor of the radio?
A. S. Popov - inventor of radio, who can be proud of our country. He first began studying the electromagnetic waves, and even was a teacher relevant courses for officers. It is Popov found the practical application of electrodynam...





















Comments (0)
This article has no comment, be the first!