Halogens: physical properties, chemical properties. The use of Halogens and their compounds
The Halogens in the periodic table located to the left of the noble gases. These five toxic, non-metallic elements are in group 7 of the periodic table. These include fluorine, chlorine, bromine, iodine and astatine. Although astatine is radioactive and only has short-lived isotopes, it behaves like iodine, and it is often referred to the Halogens. Because the halogen elements have seven valence electrons, they need only one additional electron to form a full octet. This feature makes them more active than other groups of non-metals.
General characteristics
The Halogens form diatomic molecules (of the form X2, where X is a halogen) – sustainable form of existence of Halogens in the form of free elements. The relationship of these diatomic molecules are non-polar, covalent and single. Chemical properties of the Halogens allows them to easily enter into a bond with most elements, so they never meet in the unbound form in nature. Fluoride – the most active halogen and astatine – least.
All of the Halogens form salts of group I with similar properties. In these compounds, the Halogens are present as halide anions with a charge of -1 (e.g. Cl-, Br-). The end ID indicates the presence of halide anions; for example, Cl- is called the "chloride".
In addition, the chemical properties of the Halogens allows them to act as oxidizing agents – to oxidize the metals. Most chemical reactions involving Halogens – oxidation-reduction in aqueous solution. Halogens form single bonds with carbon or nitrogen in organic compounds, where the degree of oxidation (CO) -1. When an atom of the halogen-substituted covalently-bound hydrogen atom in an organic compound, the prefix halo - can be used in a General sense, or the prefixes fluoro-, chlorine-, bromine- , iodine – specific for Halogens. Halogen elements can have a cross connection with the formation of diatomic molecules with polar covalent single bonds.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
Chlorine (Cl2) was the first halogen to be opened in 1774, then were discovered iodine (I2), bromine (Br2), fluorine (F2), and astatine (At, discovered last in 1940). The name “halo” comes from the Greek roots hal- (“salt”) and -gen (“form”). Together, these words mean “salt”, highlighting the fact that the halogen reacts with metals to form salts. Galit – the name of rock salt, a natural mineral composed of sodium chloride (NaCl). And finally, the Halogens are used in everyday life – a fluoride contained in toothpaste, chlorine disinfects drinking water, and iodine promotes thyroid hormones.
Chemical elements
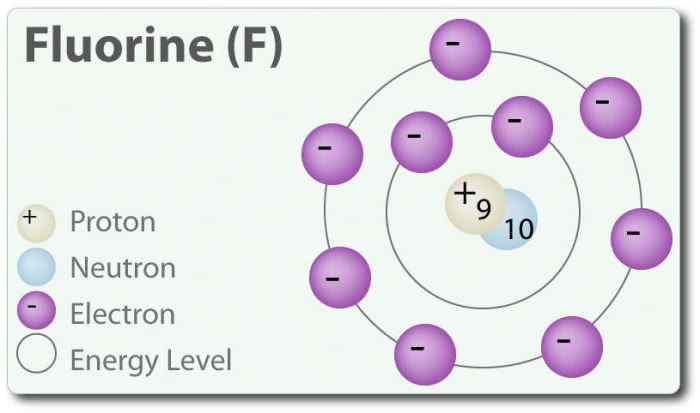
Fluoride – element with atomic number 9, represented by the symbol F. Elemental fluorine was first discovered in 1886 by isolating it from hydrofluoric acid. In the free state fluorine exists as a diatomic molecule (F2) and is the most abundant halogen in the earth's crust. Fluorine-the most electronegative element in the periodic table. At room temperature is a pale yellow gas. Fluorine also has a relatively small atomic radius. WITH it – -1, except for elemental diatomic state in which its oxidation state is zero. Fluorine is extremely reactive and interacts directly with all elements except helium (He), neon (Ne) and argon (Ar). In a solution of H2O, hydrofluoric acid (HF) is a weak acid. Although fluorine is highly electronegative, its electronegativity does not determine acidity; HF is a weak acid due to the fact that the fluoride ion is basic (pH> 7). In addition, fluorine produces very powerful oxidants. For example, fluorine can react with the inert gas xenon and forms a strong oxidizing agent xenon difluoride (XeF2). And fluorine has many uses.
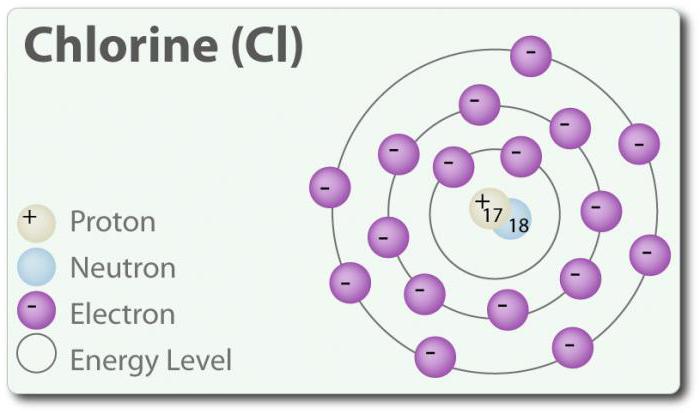
Chlorine – element with atomic number 17 and chemical symbol Cl. Discovered in 1774 by separating it from hydrochloric acid. In its elemental state, it forms a diatomic molecule Cl2. Chlorine has several FROM: -1, +1, 3, 5 and 7. At room temperature it is a pale green gas. Because the bond that is formed between two chlorine atoms is weak, molecule Cl2 has a very high ability to enter into connection. Chlorine reacts with metals to form salts called chlorides. Chloride ions are the most common ions contained in sea water. Chlorine has two isotopes: 35Cl and 37Cl. Sodium chloride is the most common connection of all the chlorides.
Brom – the chemical element with atomic number 35 and symbol Br. It was first discovered in 1826 In elemental form is diatomic bromine molecule Br2. At room temperature is a reddish-brown liquid. WITH it – -1, + 1, 3, 4 and 5. Bromine is more active than iodine, but less active than chlorine. In addition, bromine has two isotopes: 79Br and 81VG. Bromine is found as bromide salts dissolved in sea water. In recent years, the production of bromide in the world has increased significantly due to its easy availability and long life time. Like other Halogens, bromineis an oxidizer and highly toxic.
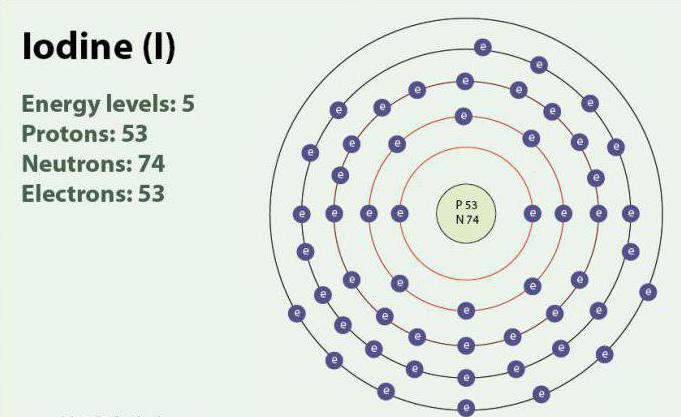
Iodine-the chemical element with atomic number 53 and symbol I. Iodine has oxidation States: -1, +1, +5 and +7. Exists as a diatomic molecule, I2. At room temperature is a solid purple color. Iodine has one stable isotope 127I. First discovered in 1811 with the help of seaweed, and sulfuric acid. At present, ions of iodine, can be isolated in sea water. Despite the fact that the iodine is not very soluble in water, its solubility may increase if you use certain iodides. Iodine plays an important role in the body, assisting in the production of thyroid hormones.
ASTAT – a radioactive element with an atomic number of 85 and symbol At. Its possible oxidation States: -1, +1, 3, 5 and 7. The only halogen that is not a diatomic molecule. Under normal conditions the metal is solid black. Astatine is a very rare element, so it is little known. In addition, astatine has a very short half-life not longer than a few hours. Received in 1940 as a result of synthesis. I believe that astatine is similar to iodine. Different metal properties.
The table below shows the structure of the halogen atoms, the structure of the outer layer of electrons.
Halo | Electron Configuration |
Fluorine | 1s2 2s2 2p5 |
Chlorine | 3s2 3p5 |
Brom | 3d10 4s2 4p5 |
Iodine | 4d10 5s2 5p5 |
Astatine | 4f14 5d10 6s2 6p5 |
This structure has an external layer of electrons determines the physical and chemical properties of the Halogens are similar. However, when comparing these items, differences were observed.
Periodic properties in the group of Halogens
The Physical properties of simple compounds of the Halogens change with increasing the ordinal number of the element. For better absorption and greater clarity, we offer you a few tables.
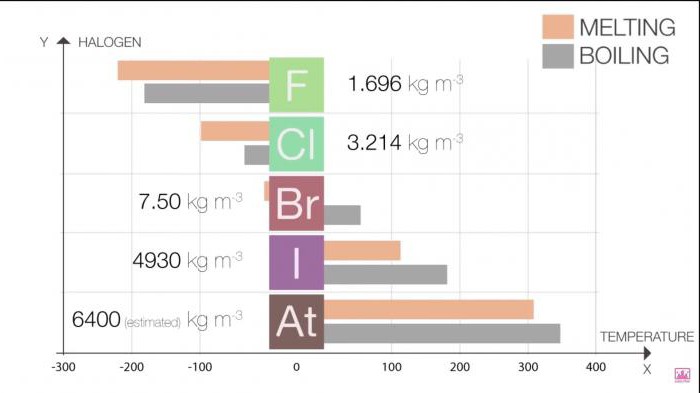
The melting Point and boiling point in the group increasing growth in the size of the molecule (F <Cl <Br <I <At). This increase means an increase in the strength of van der Waals.
Table 1. Halogens. Physical properties: melting point and boiling point
Halo | T melting (C) | T boiling point (C) |
Fluorine | -220 | -188 |
Chlorine | -101 | -35 |
Brom | -7.2 | 58.8 |
Iodine | 114 | 184 |
Astatine | 302 | 337 |
- Atomic radius increases.
The core Size increases (F < Cl < Br < I < At), as it increases the number of protons and neutrons. In addition, each period adds more energy levels. This leads to greater orbital, and hence to increase the radius of the atom.
Table 2. Halogens. Physical properties: atomic radii
Halogen | Covalent radius (PM) | Ion (X-) radius (PM) |
Fluorine | 71 | 133 |
Chlorine | 99 | 181 |
Brom | 114 | 196 |
Iodine | 133 | 220 |
Astatine | 150 |
- Ionization Energy decreases.
If the outer valence electrons are not near the nucleus, then the removal of it will not need a lot of energy. Thus, the energy required to eject the outer electrons are not so high at the bottom of the group elements, as there is more energy levels. In addition, the high ionization energy makes the element to be non-quality. Iodine and astatine display metallic properties exhibit, because the ionization energy decreases (At < I < Br < Cl < F).
Table 3. Halogens. Physical properties: ionization energy
Halo | Ionization Energy (kJ/mol) |
Fluorine | 1681 |
Chlorine | 1251 |
Brom | 1140 |
Iodine | 1008 |
Astatine | 890 ± 40 |
- Electronegativity decreases.
The Number of valence electrons in an atom increases with an increase in energy levels at progressively lower levels. The progressive electrons further from the nucleus; Thus, the nucleus and the electrons are not as attractedto each other. The increase in shielding is observed. Therefore, the Electronegativity decreases with increasing period, (At < I < Br < Cl < F).
Table 4. Halogens. Physical properties: electronegativity
Halo | Electronegativity |
Fluorine | 4.0 |
Chlorine | 3.0 |
Brom | 2.8 |
Iodine | 2.5 |
Astatine | 2.2 |
- The electron Affinity decreases.
As the size of the atom increases with increasing period, the electron affinity generally decreases (< I < Br < F < Cl). The exception – fluorine, the affinity of which is less than that of chlorine. This can be explained by the smaller size of fluorine compared to chlorine.
Table 5. Affinity of Halogens to the electron
Halo | Electron Affinity (kJ/mol) |
Fluorine | -328.0 |
Chlorine | -349.0 |
Brom | -324.6 |
Iodine | -295.2 |
Astatine | -270.1 |
- Reactivity of the elements decreases.
The Reactivity of Halogens decreases with increasing period, (At <I <Br <Cl <F). This is due to the increase in the radius of the atom with the increase of the energy levels of electrons. This reduces the attraction of valence electrons to other atoms, decreasing reactivity. This reduction also occurs due to the fall of the electronegativity with increasing period, which also reduces the attraction of electrons. In addition, with the increasing size of the atom decreases and oxidative capacity.
Inorganic chemistry. Hydrogen + Halogens
The Halide formed when a halogen reacts with another, less electronegative element with the formation of the binary compounds. Hydrogen reacts with Halogens to form halides of the form HX:
- Hydrogen fluoride HF;
- Hydrogen chloride HCl;
- Bromovalerate HBr;
- Yodovidona HI.
The hydrogen Halides dissolve easily in water to form halogenoalkanes (hydrofluoric, hydrochloric, bromatological, itestosterone acid). The properties of these acids are given below.
Acid formed by the following reaction: HX (aq) + H2O (l) → X- (aq) + H3O+ (aq).
All halomonadaceae form strong acids, except HF.
The acidity of the hydrohalic acids increases: HF <HCl <HBr <HI.
Hydrofluoric acid can etch glass and certain inorganic fluorides for a long time.
It May seem counterintuitive that HF is the weakest halogenation acid because fluorine has the highest electronegativity. However, the bond N-F is very strong, causing acid is very weak. A strong bond is determined by the short length of the link and a large dissociation energy. Of all the hydrogen halides, HF has the shortest length for communication and the greatest energy of bond dissociation.
Halogen oxoacid
Halogen oxoacid represent the acid with the atoms of hydrogen, oxygen and Halogens. Their acidity can be determined by analyzing the structure. The halogen oxoacid is shown below:
- Hypochlorous acid is HOCl.
- Hydrochloric acid HClO2.
- Chloric acid HClO3.
- Perchloric acid HClO4.
- Bromoviridae acid is HOBr.
- Vranovaca acid HBrO3.
- Bromine acid HBrO4.
- Innovatica acid HOI.
- Alpha-iodic acid HIO3.
- Medioda acid HIO4, H5IO6.
In each of these acids, the proton linked to the oxygen atom, therefore, the comparison of bond lengths of protons are useless. The dominant role is played by the electronegativity. The activity of the acid increases with the increase in the number of oxygen atoms associated with the Central atom.
Appearance and state of matter
The Main physical properties of the Halogens can be briefly expressed in the following table.
State of matter (at room temperature) | Halo | Appearance |
Solid | Iodine | Purple |
Astatine | Black | |
Liquid | Brom | Red-brown |
Gaseous | Fluorine | Pale yellow-brown |
Chlorine | Pale green |
Explanation of appearance
The color of the Halogens is a result of absorption of visible light by molecules, which causes excitation of electrons. Fluorine absorbs violet light, and therefore looks light yellow. Iodine, on the contrary, absorbs yellow light and it looks purple (yellow and purple – complementary color). The colour of Halogens become darker with increasing period.
In closed containers of liquid bromine and solid iodine are in equilibrium with their vapors, which can be observed in the form of a colored gas.
Although the color of astatine is unknown, it is assumed that it needs to be darker iodine (i.e. black) in accordance with the observed pattern.
Now, if you are asked: “describe physical properties of Halogens”, you will have something to say.
The oxidation state of Halogens in compounds
The Degree of oxidation is often used instead of the concept of "valence of the Halogens". As a rule, the oxidation state of -1. But if the halogen is associated with the oxygen or with another halogen, it may take another condition: the oxygen has -2 priority. In the case of two different halogen atoms bonded together, the more electronegative atom prevails and takes WITH -1.
For Example, the chloride of iodine (ICl) chlorine has a -1, and the iodine +1. Chlorine is more electronegative than iodine, so it is WITH -1.
In bromine acid (HBrO4), oxygen has WITH -8 (-2 x 4 atoms = -8). Hydrogen has a total oxidation state +1. The addition of these values gives WITH -7. As WITH the end connections should be zero, then the bromine is +7.
The Third exception to the rule is the oxidation state of halogen in elemental form (X2), where it FROM zero.
Halo | In connection |
Fluorine | -1 |
Chlorine | -1, +1, +3, +5, +7 |
Brom | -1, +1, +3, +4, +5 |
Iodine | -1, +1, +5, +7 |
Astatine | -1, +1, +3, +5, +7 |
Why fluorine is always -1?
Electronegativity increases with increasing period. Therefore, fluorine has the highest electronegativity of all elements, as evidenced by its position in the periodic table. Its electronic configuration is 1s2 2s2 2p5. If fluoride gets one more electron, outermost p-orbitals are completely filled and are a complete octet. Since fluorine has high electronegativity, it can easily take an electron from a neighboring atom. Fluorine in this case isoelectronic inert gas (eight valence electrons), all outer orbitals are filled. In this state, the fluorine is much more stable.
Production and use of Halogens
In nature, the Halogens are in a state of anions, so the free Halogens produced by oxidation by electrolysis or by using oxidizing agents. For example, chlorine is produced by the hydrolysis of the salt solution. The use of Halogens and their compounds are diverse.
- Fluoride. Despite the fact that fluorine is very reactive, it is used in many industries. For example, it is a key component of polytetrafluoroethylene (Teflon) and other fluoropolymers. CFCs are organic chemicals that were formerly used as refrigerants and propellants in aerosols. Their use ceased due to their possible impact on the environment. They were replaced by hydrochlorofluorocarbons. Fluoride is added to toothpaste (SnF2) and drinking water (NaF) to prevent tooth decay. This halogen is contained in the clay used for the production of certain types of ceramics (LiF) is used in nuclear energy (UF6) to obtain the antibiotic fluoroquinolones, aluminum (Na3AlF6), for isolation of high voltage equipment (SF6).
- Chlorine also found diverse applications. It is used to disinfect drinking water and swimming pools. Sodium hypochlorite (NaClO) is the main component of bleach. Hydrochloric acid is widely used in industry and laboratories. Chlorine is present in polyvinyl chloride (PVC) and other polymers, which are used for wiring insulation, pipes and electronics. In addition, chlorine has proved useful in the pharmaceutical industry. Medicinal products containing chlorine, are used to treat infections, allergies and diabetes. The neutral form of the hydrochloride-a component of many drugs. Chlorine is also used for sterilization of hospital equipment and disinfection. In agriculture, the chlorine is a component of many commercial pesticides: DDT (dichlorodiphenyltrichloroethane) was used as an agricultural insecticide, but its use was discontinued.

- Bromine, due to its incombustibility, it is used to suppress combustion. It is also found in methyl bromide, a pesticide used for storage of crops and the suppression of bacteria. However, excessive use of methyl bromide was discontinued because of its impact on the ozone layer. The bromine used in the production of gasoline, film, fire extinguishers, medicines for the treatment of pneumonia and Alzheimer's disease.
- Iodine plays an important role in the proper functioning of the thyroid gland. If the body does not receive enough iodine, the thyroid gland. Prevention of goiter, the halogen is added to table salt. Iodine is also used as antiseptic. The iodine contained in solutions used forcleaning of open wounds and also in disinfectant sprays. In addition, the silver iodide is important in photography.
- Astatine – radioactive and rare earth halogen, so have never used. However, it is thought that this element can help iodine in regulating thyroid hormones.
Article in other languages:

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
The Reign Of Alexei Mikhailovich Quietest. The order of Secret Affairs
the establishment of the order of Secret Affairs (the year of the formation approximately 1653-y), initiated by Alexei Mikhailovich Quietest, had two goals. On the one hand, it was used as a private office of the Emperor. On the o...
Captain is a rank in the Cossack army
the captain – a rank in the Cossack army. First it was called the assistant to the commander, later captain can be equated with the captain or the captain. What does this word mean?EtymologyAccording to one version, Esaul &n...
Tanya Savicheva: biography, blockade diary, and interesting facts
the Usual Leningrad girl Tanya Savicheva has become world famous thanks to its diary, which she led to the 1941 – 1942 during the siege of Leningrad. This book has become one of the main symbols of those terrible events.Plac...
In which cases is a comma? Commas in sentences: rules
a Comma – the most simple and prosaic, but at the same time and the most insidious sign. Production entails understanding how it is constructed and structured, and what meanings appear and disappear, if it is wrong to put a ...
Atomic absorption spectrometer MGA
In this article we will discuss the concept of atomic absorption spectrometer and field of study in which the instrument is used. We also pay attention to the General definition of analytical tools, various historical data and pri...
How are the activities and needs of the person? Need, activity, motive activity
how are associated activities and needs, said a lot. There are entire scientific papers dedicated to this topic. It is really very interesting and diverse. All of it is difficult to discuss, because the essence for the unknown cre...





















Comments (0)
This article has no comment, be the first!