Proper pharmacy practice. The rules of good pharmacy practice medicinal products (order of Ministry of health of the Russian Federation No. 647н)
About approval of rules of good pharmacy practice in the pharmaceutical sphere were talking back in 2016. It was widely believed that this document will become a key industry in the act, 2017 and So it happened. Let us consider briefly the contents of act Good pharmacy practice review. 
General information
Order 647н, containing the new order was registered by Ministry of justice on 9 January 2017 This document came into effect on March 1 of the year indicated.
Order 647н is a set of instructions, the failure of which shall entail the appropriate consequences, including administrative responsibility according to KoAP. Other orders, decrees, laws this document is, of course, does not change. The order of Ministry of health, however, accumulates a number of their provisions. They are all now contained in a single legal act.
Rules of good pharmacy practice: discussion
Before the introduction of the document into action it was felt that it will be the most used instrument in the pharmaceutical industry. And pharmacy managers and pharmacists, and pharmacists, and other employees will open the Rules of good pharmacy practice to specify how to make a particular product, how to organize paperwork how to consult the buyer and so on. Simply put, it was expected that the act will be a benefit No. 1 in the pharmacies.
The Rules of good pharmacy practice there are new guidelines and regulations. Their use is, of course, a few will change the daily activities of pharmacy organizations.
Fixed The Order of Ministry of health Rules of good pharmacy practice contain, among other things, a detailed description of the actions, mechanisms and processes of the pharmacies. For example, they contain details of the implementation of acceptance inspection of the products.
Recommended
Calculation and payment of sick leave
Sick Pay provided by the legislation of the Russian Federation, in particular TC and FZ No. 255. In addition, some rules are governed by the provisions of the civil code. Any employee upon the occurrence of a certain disease should contact a health f...
Employee certification for compliance with the post: purpose, procedure, result
Employers perceive the order of certification of employees as a formality. Regulations intended for commercial organizations, were not issued. Certification is required only for employees of the organizations designated in the laws of the spheres, le...
Registration of vehicle: procedure, sample application, certificate
Every person who buys a car needs to do its registration in the traffic police. It is necessary when purchasing new or used cars, as well as no matter whether the seller of natural persons or legal entities. Check the vehicle is in the traffic police...
International practice
It is Worth to say that work on sets of Rules is fairly long. So, in 1993 the IFF (international pharmaceutical Federation) has developed a document, the name of which in Russian translates as "Good pharmacy practice".
In 1997 and 2001 this document was processed. In the revision of the "Good pharmacy practice", were not only international, but also who.
To say that the NAP was not any specific guidance. The document was not a detailed description of all procedures and aspects of pharmacy work. "Proper pharmacy practice" – a common base the scheme on the basis of which had developed the regulations in different countries taking into account the specifics of a state. National NAB, in turn, must be detailed. 
Prerequisites to the adoption of the document
Implementation of the Rules of good pharmacy practice is due, according to experts, two reasons.
First and foremost the Ministry of health together with the health Ministry has intensified its efforts to improve the regulatory framework of the pharmaceutical sector.
Second, experts believe that the emergence of Rules of good pharmacy practice in the Russian Federation related to the participation in the EEU. The fact that Russia's partners in this organization have long had their NAP. One of directions of work of the Ombudsman institutions of the EEU is to bring into a single form of pharmacological legislation of member countries.
Structure
The Rules of good pharmacy practice consist of 8 sections:
- The First and the second – General provisions and terms.
- Third, fourth – reveal the peculiarities of the system of quality management and control processes.
- Fifth – highlights the issues related to resources (personnel, equipment, infrastructure, and so on).
- Sixth – present description of the different processes in the framework of the activities of the pharmacy organization. For example, the specification of the operations on purchase, acceptance, storage, sale of goods.
- The Seventh section is devoted to introspection – the evaluation of the activities of the pharmacy.
- In eighth – refers to continuous improvement.
New terminology
The new Rules of good pharmacy practice explores the concept of "pharmaceutical service". It understands the service provided by the pharmacy organization and aims to ensure the needs of the buyer in medicines and other products of pharmaceutical assortment. In the framework of providing consumers and health care providers should obtain information about the presence, storage, use of products.
The Consultation is aimed at ensuring responsible self-medication. Under it, in turn, need to understand reasonable use by the purchaser nonprescription Drugs. According to the Rules NAP, they should be used for the prevention of health disorders of mild to medical care. From this we can conclude that self-application of prescription drugs, for example antibiotics, is regarded as an irresponsible self-medication.
In article 2.4 deals with the concept of "pharmaceutical products range." It is believed that this term was first fixed on a normative level. However, innovation can be called formal, because the definition is almost completely duplicates the paragraph 7article 55 "About Retail". The pharmaceutical range Listed in it in some detail.
Showcase
One of the novels "Good pharmacy practice" is the provision on the storage of medicines. It is present in the section that contains information about the equipment.
Attention should be paid to the wording that allowed storage of prescription drugs on display in open, glass cabinets, if consumers do not have physical access. This provision caused a lot of controversy even before the adoption of Standards of good pharmacy practice.
In the international practice, three approaches to the release and display of prescription medicines. In some countries, leave is solely at the recipes, and showcases such funds are laid. In other States there are no restrictions in this matter.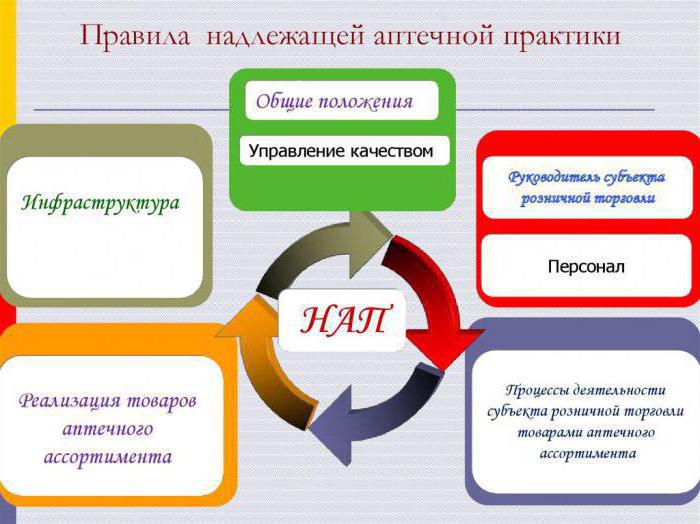
The provisions of the "Good pharmacy practice" in Russia experts have called the third approach. You must follow the terms:
- Strict observance of the order of issue of prescription drugs.
- Display in the window is allowed if guaranteed no buyer's access to medicines.
Specify the open glass cabinets aimed, among other things, to pharmacy organizations did not apply penalties for funds that are on display behind the pervostolnik (pharmacist serving consumers) who do not have access to pharmacies, but addressed to him. Auditors often consider glass "facade" showcase, as it is visible from the hall. Accordingly, to pharmacy claims. Now everything will depend on strict adherence to the set conditions.
It Should be noted that the wording "to keep" does not mean "must".
Experts predicted that the probability of the extension of the calculations in prescription pharmacies is very small. The fact that some organizations do not have sufficient space, the employees of other structures tend to minimize contacts with consumers who saw the medicine in the window and require or ask you to sell it without a prescription.
Reception products
This process is detailed in the new Rules. Experts and pharmaceutical industry representatives respond positively to this innovation.
The Procedure for acceptance of the goods the subject of the article 6.2. It explains in detail inspection. In particular, there are guidelines on what details the primary and secondary packaging, documentation, labeling is necessary to pay attention.
The acceptance Process detailed not only for pharmaceutical substances and medicinal preparations. Detailed and of Supplements, diet products, baby, health food, tools, and items for childcare, cosmetic products, mineral waters, medical products. 
Experts recommend to make a printout of the rules inspection and keep it in a visible place as a visual aid.
Counselling
In article 6.4 says that the sale of products in pharmacies involves not only direct the release and implementation, but also providing information within the scope of pharmaceutical workers. Of particular note are the following provisions of law:
- If requested By the buyer, the employees of the pharmacy organization is to acquaint the citizen with the certificate or Declaration of conformity are interested in his product.
- Sales of products non-medicinal purpose can be accomplished by personnel without pharmaceutical training.
- To provide consultancy and other pharmaceutical services it is advisable to allocate an area for a personal interview. This can be done by installing a special limiter, applying bright color border to the expectations the organization places, etc.
According to experts, this provision, of course, correct. After all, every buyer has the right to consult in the framework of private discussions on matters of their own health, including with the pharmacist. Experts emphasize that these rules are Advisory, not mandatory. The fact that in the framework of current legislation and the current pharmacy practice, far not all pharmacies may allocate such zones technically, not everywhere it is appropriate.
In the small pharmacies are no place for large objects, on the contrary, the space allows you to hold a private conversation without the office of the special zone.
Application to article 6.4
Only two of Them. App fix simple scheme of counselling for cases when the user:
- Asks the item.
- Needs to be consultation on symptoms. For example, a man goes into a pharmacy and says his stomach hurts, runny nose, or something else.
In the Rules it is noted that for each symptom, the pharmacy must have a separate survey design. However, the NAP does not explain where to take the sample.
Controversial issue
Experts focus on one provision of article 6.4. It provides that a pharmaceutical employee is obliged to make every effort to ensure that the buyer, made the decision to purchase drugs, has formed a sufficient understandingabout:
- Operation;
- Duration and method of application;
- Probable adverse reactions;
- Rules for home storage;
- Cost;
- Combined with other medicines and foodstuffs;
- Contraindications;
- Need to consult a doctor if symptoms persist;
- Failing to return medication of poor quality, and so on.
Of Course, most of this information is present in the instructions to the tool. However, about it in article 6.4 does not say. 
The analysis of language there are many questions. For example, what it means to "make every effort"? How to measure the "adequacy of representation" of the buyer about the product?
Experts note the vagueness, the subjectivity of the wording. Some experts suggest that these gaps give another reason regulatory agencies to apply to for pharmacy sanctions.
Pervostolnik, of course, able to answer all customer questions (within his competence, of course), provide accurate information, and so on. However, the pharmacist even with all this, can not guarantee the formation of "adequate representation" to the client about the product. And suddenly people were not listening very carefully or didn't get enough sleep today? In addition, perhaps consumers in General have come in the pharmacy to submit a claim.
In addition, it is necessary to understand what the detailed consultation may take some time. How then to deal with other customers in the queue? After all, they too are entitled to "adequate representation" on the interest of their goods.
Staff
To meet the requirements prescribed by the Rules, the head of the pharmacy is required to approve the staffing. It must contain:
- The names of the positions, occupations, professions, information about qualification.
- Data on the number of staff units.
- Information about the PAYROLL (the wage Fund).
Each employee should be familiar with his duties and rights under the painting.
For activities that affect product quality may not be staff with the necessary qualifications and experience.
Actually, all these rules are present in other regulations, standards, etc. 
Programme
It is implemented for newly hired employees. After the program checked from time to time knowledge, skills, experience.
Programme includes:
- Induction.
- Training in direct place of work (primary and secondary).
- Updating of knowledge of regulations on circulation of medicines, the health of the population, protection of consumer rights, the procedure for the provision of pharmaceutical services, including counseling about the use of medical products in the home; hygiene.
- Develop skills of communication and conflict prevention.
- Training on health and safety (health and safety).
Requirements for experience and qualifications of the Director and employees of a pharmacy laid down in the Statute on licensing of pharmaceutical activities.
The Issues of preparation
The Head of a pharmaceutical organization provides training on the rules of vacation:
- Medicines for medical use;
- Narcotic/psychotropic drugs;
- Medicines in respect of which is subject-quantitative accounting;
- Medicines containing small amounts of narcotic compounds.
During the preparation of the staff also addresses issues relating to:
- Storage of the recipes.
- Compliance with the requirements of the minimum range.
- Use limits retail markups to selling prices of medicines included in the list of essential drugs order form of of their cost.
- Compliance with storage and transportation of medicines.
- The requirements when working with a falsified, counterfeit, poor quality goods.
- Compliance with the restrictions provided for pharmacists in discharging their professional duties.
- Improve the knowledge about medicines, including generic and interchangeable means the ability to provide comparative information on products and prices.
- Methods of processing of information received from consumers regarding the use of drugs, side effects, communicating this information to stakeholders.
Assessment activities
First and foremost, it is performed by the head of the pharmacy organization. The evaluation is aimed at testing the completeness of compliance with the requirements laid down in the Rules of NAP, to determine corrective actions.
Analysis of the issues relating to personnel, premises, equipment, observance of rules of sale of products of pharmaceutical assortment, documentation, events, working with the suggestions and feedback of consumers, activities to detect counterfeit, adulterated, substandard goods, internal audit, performed by the head in accordance with the schedule, approved in the prescribed manner. 
Internal audit
He must be independent and thorough. Internal audit is performed by persons from among the staff authorized by the head of the pharmacy organization. Allowed third party entities on a contractual basis.
The results of the inspection should be documented. The documentation shall include all information obtained in the course of the audit, as well as proposals for corrective measures, if there is a need for them.
The Measures taken by the audit also recorded the acts.
The audit also finds shortcomings in the process of complying with the requirements of legislation and the formulation of recommendations for preventive and corrective actions.
The internal audit program should take into account the results of previous audits, including those conducted by regulatory authorities.
The Entity appointed responsible for the audited activity of a pharmacy, shall ensure the prompt execution of preventive and corrective measures.
Article in other languages:
AR: https://tostpost.com/ar/the-law/865-647n.html
HI: https://tostpost.com/hi/the-law/865-647n.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
The primary extinguishing media: General requirements
In each building must be the primary means of fire extinguishing. Instructions for fire safety in any organization has paragraphs explaining how to act during a fire and what to take measures to prevent the spread of fire. Persons...
How to obtain a residence permit in the EU?
Developed economy, high level of culture, advances in medicine, social insurance, and, moreover, clear rules – main components of residence in the European countries. So many question arises about how to obtain a residence p...
St. 81 OF THE LC RF. Termination of employment contract on the initiative of the employer
the Legislation provides for the grounds on which an employer may terminate an employment contract. They are defined in article 81 of the LC RF. Consider the rule in more detail. FoundingDismissal under article 81 TK the Russian F...
Time off for previously worked time. How to take the day off?
the Majority of working people in the service from 8.00 to 17.00 daily during weekdays. Unfortunately, the majority of agencies working on the same schedule, which means that employees sometimes have to leave work to solve their p...
2 group disability working or not? Benefits and payments
This article is useful reading not only disabled people but also healthy people. Few people think about the fact that becoming disabled can potentially every. Can circumstances so that a few minutes ago a healthy man became a pers...
How to write application for withdrawal of resignation: sample
Labor laws allows employee to withdraw his resignation within two weeks from the date of its transfer to the head of the organization. The employee may do so at any time before the expiration of the specified period. If the employ...






















Comments (0)
This article has no comment, be the first!