Now - 10:15:09
The formula of oxygen. Periodic table of elements - oxygen
The Chemical element oxygen is in the second period of the VI-th main group legacy short version of the periodic table. Under the new standards numbering — is the 16th group. The decision was made by IUPAC in 1988. The formula of oxygen as a simple substance — O2. Consider its basic properties, role in nature and agriculture. Let's start with the characteristics of the entire group of the periodic system, headed by oxygen. The element differs from its related chalcogens, and the water is different from hydrogen compounds of sulphur, selenium and tellurium. The explanation for all the differences you can find, only to learn about the structure and properties of the atom.
The Chalcogens-oxygen related items
Similar properties of atoms form the same group in the periodic table. Oxygen tops the family of chalcogens, but differs from them in several properties.
The Atomic mass of oxygen — the founder of the group — is 16. E. M. Chalcogens in the formation of compounds with hydrogen and metals show its usual oxidation state: –2. For example, in the composition of the water (H2O) the oxidation number of oxygen is equal –2.
The typical Composition of the hydrogen compounds of the chalcogens corresponds to the General formula: H2R. the dissolution of these substances to form acids. Only the hydrogen compound of oxygen — water — has special properties. According to the findings of scientists, this unusual substance is a very weak acid and very weak base.
Sulfur, selenium and tellurium have a positive oxidation number (+4, +6) compounds with oxygen and other nonmetals with high electronegativity (EO). The composition of the oxides chalcogens reflect the General formulas: RO2, RO3. The corresponding acid have the composition: H2And RO3, H2And RO4.
Recommended
"Knowledge is light and ignorance is darkness": the value, meaning and alternatives
There are some sayings that would seem to need no explanation, such as “teaching & ndash; light and ignorance – darkness”. But some still do not understand their meaning. But not only for such people is written by our article. I...
What was invented by Mendeleev for the army. The history and fate of the invention
D. I. Mendeleev was a brilliant Russian scientist-polymath, who made many important discoveries in various fields of science and technology. Many people know that he is the author of “Fundamentals of chemistry" and the periodic law of chem...
The origin of the Slavs. The influence of different cultures
Slavs (under this name), according to some researchers, appeared in the story only in 6 century ad. However, the language of nationality bears the archaic features of the Indo-European community. This, in turn, suggests that the origin of the Slavs h...
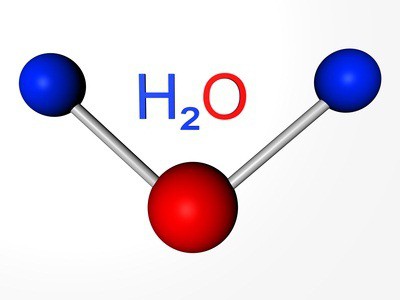
The Elements are simple substances: oxygen, sulfur, selenium, tellurium and polonium. The first three representatives of non-manifest properties. The formula of oxygen — O2. Allotropic modification of the same element - ozone (O3). Both versions are gases. Sulfur and selenium — the solid nonmetals. Tellur — metallocene substance, a conductor of electric current, polonium — the metal.
The Oxygen — the most abundant element
The Total content of atoms of the element in the earth's crust is about 47 % (by weight). Oxygen occurs in the free form and in the composition of numerous compounds. Simple substance the formula of which is O2, is in the composition of the atmosphere, comprising 21% of air (by volume). Molecular oxygen dissolved in the water contained between the soil particles.
We already know that there is another kind of existence of the same chemical element in the form of simple substances. This ozone-gas that forms at an altitude of about 30 km from the earth's surface layer, often called the ozone shield. The bound oxygen included in the water molecules, the composition of many rocks and minerals, organic compounds.
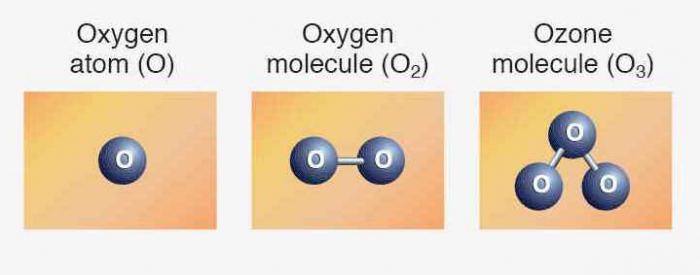
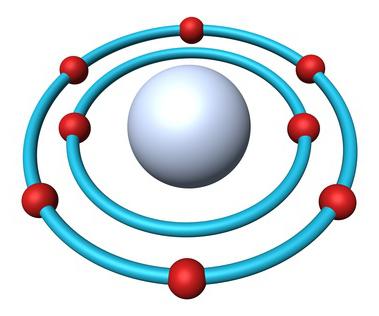
Structure of the atom of oxygen
Periodic table of elements contains the full information about the oxygen:
- Number 8.
- The Charge of the nucleus — the +8.
- Total number of electrons — 8.
- Electronic formula of oxygen — 1s22s22p4.
In nature there are three stable isotopes that have the same ordinal number in the periodic table, identical to the composition of protons and electrons but different number of neutrons. Isotopes are denoted by the same symbol — O. For comparison, below is the chart showing the composition of the three isotopes of oxygen:
Properties of oxygen — chemical element
In the 2P-sublevel of an atom has two unpaired electrons, which explains the appearance of oxidation States –2 and +2. Two paired electrons can not disconnect to the degree of oxidation increased to +4, as in sulfur and other chalcogens. The reason — the lack of free tier. Therefore, in the compounds of the chemical element oxygen does not show the valency and oxidation state equal to group number in a short version of the periodic system (6). His usual oxidation number is equal –2.
Only in compounds with fluorine, oxygen exhibits an uncharacteristic positive oxidation state +2. The value of EO of two strong non-metals different: EI (On) = 3,5; EO (F) = 4. As a more electronegative element, fluorine is stronger than holds its own electrons and attracts the valence particles on the outer energy level of an atom of oxygen. Therefore, in the reaction with fluorine, oxygen is the reducing agent gives electrons.
The Oxygen — a simple substance
The English scientist J. Priestley in 1774, in the course of experiments has allocated gas from the decomposition of mercury oxide. Two years earlier, the same substance in the pure form received by K. Scheele. Only a few years later the French chemist A. Lavoisier established that the gas enters in the composition of the air, studied the properties. The chemical formula of oxygen — O2. Reflect in the record the composition of the substance the electrons involved in the formation of nonpolar covalent bonds — O::O Replaceeach binding a few e-one feature: About=O. This formula of oxygen shows that the atoms in the molecule connected between two shared pairs of electrons.

Perform simple calculations and determine what is the relative molecular mass of oxygen is: Mr(O2) = Ar(O) x 2 = 16 x 2 = 32. For comparison, Mr(air.) = 29. The chemical formula of oxygen differs from the formula of ozone per atom of oxygen. So, Mr(O3) = Ar(O) x 3 = 48. Ozone is 1.5 times heavier than oxygen.
Physical properties
Oxygen is a gas without color, taste and odor (at normal temperature and pressure equal to atmospheric). The substance is slightly heavier than air; soluble in water, but in small quantities. The melting point of oxygen is negative and amounts to-218,3 °C. the point at which the liquid oxygen becomes gaseous again, is its boiling point. For molecules O2 the value of this physical quantity reaches –182,96 °C. In liquid and solid state oxygen gains a light blue color.
Getting the oxygen in the lab
When heated, oxygen-containing compounds, for example potassium permanganate, is allocated colorless gas that can collect in the flask or vial. If you add in pure oxygen burning torch, it burns more brightly than in the air. Two other laboratory method of obtaining oxygen - decomposition of hydrogen peroxide and potassium chlorate (potassium chlorate). Consider the scheme of the device that is used for the thermal decomposition.
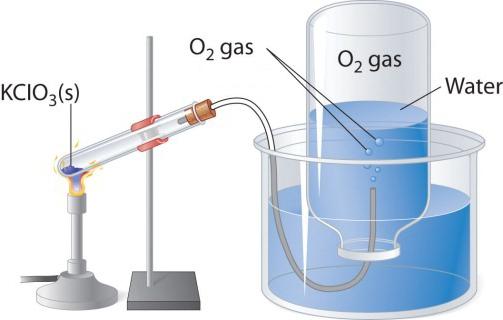
In a test tube or a round bottom flask should pour a little potassium chlorate, close the tube with the vapour tube. The opposite end should be sent (under water) in the tilted upside down flask. The neck should be lowered in a wide beaker or crystallizer filled with water. When heated, the test tube with potassium chlorate salt oxygen. In the vapor tube it enters the flask, displacing the water from it. When the bulb fills with gas, it is covered under the water tube and turn it over. Obtained in this laboratory experiment the oxygen can be used to study chemical properties of simple substances.
Burning
If in the lab is the burning of substances in oxygen, it is necessary to know and observe fire-prevention rules. Hydrogen burns instantly in air, and mixed with oxygen in the ratio 2:1, it is explosive. Burning substances in pure oxygen is much more intense than in the air. Due to this phenomenon the composition of the air. The oxygen in the atmosphere is a little more than 1/5 of (21%). Burning is the reaction of substances with oxygen, which are formed of different products, mostly oxides of metals and nonmetals. Flammable mixtures of O2 with flammable substances, in addition, the resulting compounds can be toxic.
Burning a regular candle (or match) is accompanied by formation of carbon dioxide. The following experiment can be carried out at home. If the substance to burn under a glass jar or large glass, the burning will stop as soon as I used up all the oxygen. Nitrogen does not support respiration and combustion. Carbon dioxide — a product of oxidation — no longer reacts with oxygen. The transparent lime water allows to detect the presence of carbon dioxide after burning the candle. If you pass the combustion products through the calcium hydroxide, the solution becomes cloudy. There is a chemical reaction between lime water and carbon dioxide, it turns out insoluble calcium carbonate.

The production of oxygen on an industrial scale
The Most cheap process, which may result in getting loose from air molecules O2, not associated with conducting chemical reactions. In industry, for example, in steel mills, the air is at low temperature and high pressure liquefied. Such important components of the atmosphere like nitrogen and oxygen boil at different temperatures. Share air mixture during the gradual heating to the normal temperature. First stand molecules of nitrogen, then oxygen. Method of separation based on different physical properties of simple substances. The formula is simple substance of oxygen is the same as it was before the cooling and liquefaction of air, — O2.
As a result of some reactions of electrolysis also releases oxygen, it is collected on the relevant electrode. Need gas industrial, construction enterprises in large volumes. Oxygen demand is constantly growing, especially in need of the chemical industry. Store the received gas for industrial and medical purposes in steel cylinders, label. Containers with oxygen are painted in blue or light blue color, to distinguish it from other liquefied gases-nitrogen, methane, and ammonia.

Chemical calculations according to the formula and equations of reactions with participation of molecules O2
The Numerical value of the molar mass of oxygen coincides with another value — relative molecular mass. Only in the first case are units of measurement. Briefly the formula of a substance of oxygen and its molar mass should be written as: M(O2) = 32 g/mol. Under normal conditions prayany gas corresponds to a volume of 22.4 L. So, 1 mole of O2 — it's 22.4 l substance 2 mol O2 — 44,8 L. the equation of the reaction between oxygen and hydrogen can be noted that the interact 2 moles of hydrogen and 1 mole of oxygen:
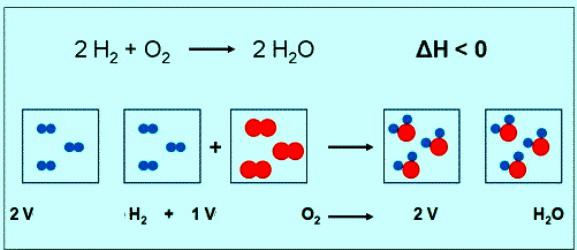
If the reaction involved 1 mole of hydrogen, the volume of oxygen will be 0.5 mol • 22.4 l/mol = 11,2 L.
The Role of molecules O2 in nature and human life
Oxygen is consumed by living organisms on Earth and involved in the nutrient cycle more than 3 billion years. This is the main substance for respiration and metabolism, with the help of the decomposition of the molecules of nutrients, synthesized necessary for organisms energy. Oxygen is constantly consumed on Earth, but its reserves are replenished through photosynthesis. Russian scientist K. Timiriazev believed that due to this process there is still life on our planet.

The role of oxygen in nature and economy:
- Absorbed in the process of respiration of living organisms;
- Participates in photosynthetic reactions in plants;
- Is part of organic molecules;
- The processes of decay, fermentation, rusting occur with the participation of oxygen, acting as oxidizer;
- Can be used to obtain valuable products of organic synthesis.
Liquefied oxygen cylinders used for cutting and welding metals at high temperatures. These processes are carried out at mechanical engineering factories, transport and construction enterprises. For work under water, underground, at high altitude in the vacuum of space people need in the molecules O2. Oxygen tanks are used in medicine for the enrichment of the composition of the air that is inhaled people who are sick. Gas for medical purposes differs from technical almost no impurities, odorless.

The Oxygen — the ideal oxidizer
Known oxygen compounds with all chemical elements of the periodic table, except the first representatives of the family of noble gases. Many substances directly enter into reaction with the atoms On, except for the Halogens, gold and platinum. Of great importance are phenomena involving oxygen, which are accompanied by light and heat. Such processes are widely used in household and industry. In metallurgy the interaction of the ore with oxygen is called burning. Pre-crushed ore is mixed with oxygen enriched air. At high temperatures, the recovery of metals from sulphides to simple substances. So get the iron and some nonferrous metals. The presence of pure oxygen increases the rate of technological processes in different branches of chemistry, technology and metallurgy.
The Advent of cheaper ways of obtaining oxygen from air by separation of components at a low temperature stimulated the development of many areas of industrial production. Chemists think of molecules O2 And o atoms Of the ideal oxidizing agents. It is a natural material, they are recurring in nature, do not pollute the environment. In addition, chemical reactions involving oxygen most often conclude with a synthesis of another natural and safe product — water. The role O2 in the disposal of toxic industrial waste, the purification of water from contaminants. In addition to the oxygen used for disinfection of its allotropic modification-ozone. This simple substance has a high oxidative activity. During the ozonation of water decompose pollutants. Ozone also has a detrimental effect on pathogenic microflora.
Article in other languages:
KK: https://tostpost.com/kk/b-l-m/20346-formulasy-otteg-mendeleevt-periodty-kestes---otteg.html
PL: https://tostpost.com/pl/edukacja/20321-formu-a-tlenu-uk-ad-okresowy-mendelejewa---tlen.html
TR: https://tostpost.com/tr/e-itim/20355-form-l-oksijen-periyodik-tablo-periyodik---oksijen.html
UK: https://tostpost.com/uk/osv-ta/20338-formula-kisnyu-per-odichna-tablicya-mendel-va---kisen.html

Alin Trodden - author of the article, editor
"Hi, I'm Alin Trodden. I write texts, read books, and look for impressions. And I'm not bad at telling you about it. I am always happy to participate in interesting projects."
Related News
Text types and their characteristics
Each text consists of connected sentences, or paragraphs, which are combined in a single unit a specific theme and main idea. All the sentence have a semantic relationship, which builds the hierarchy of the parts in meaning, impor...
The perfect and the imperfect form of the verb
the verb aspect in the Russian language, is a consistent grammatical category, as is found in all forms of this part of speech. The same reason allows it to be regarded as predicative categories, as it can be realized not on...
Where the Arctic, Antarctic and Antarctica: the main differences and interesting facts
Where is the Arctic and Antarctic? And what is the difference between these areas of Land from each other? This question puzzles many people, even if they conscientiously studied geography in school. Answer it will help our articl...
A brief history of the Czech Republic
History of the Czech Republic has more than a dozen centuries. The lands of this state lying in the Central part of the European continent, has always played an important task. Starts the history of the earth, which is the Czech R...
The composition of oil and oil properties
Oil – it's oily and flammable liquid that is spread in the area of the sedimentary shell of the earth's crust. It is one of the most important minerals for humans. Oil is a very complex mixture zilanov, arenes, and alk...
Where is the Crimea - the pearl of Europe?
Where is the Crimea, it is interesting to know many. Most people have heard of this Peninsula, and knows about how many minutes it differs. Those who are fortunate enough ever to come to the Crimea, eager to come back, and that's ...






















Comments (0)
This article has no comment, be the first!